.
Condición fitosanitaria: Presente solo en algunas áreas del país
Grupo de cultivos: Oleaginosas
Especie hospedante: Soja (Glycine max)
Rango de hospedantes: amplio rango de hospedantes alternativos o secundarios (roya atípica). En el campo, P. pachyrhizi infecta el tejido foliar de un amplio rango de leguminosas (al menos 31 especies en 17 géneros). La infección de 60 especies adicionales en otros géneros se ha logrado en condiciones de laboratorio (Goellner et al., 2010; Gupta et al., 2022). En total, se sabe que infecta a más de 95 especies de más de 42 géneros (Slaminko et al., 2008; Bettgenhaeuser et al., 2014).
Epidemiología: policíclica, subaguda.
Ciclo: no se conoce ningún hospedante intermediario. Las basidiosporas no pueden infectar la soja.
Etiología: Hongo. Biotrófico
Agente causal: Phakopsora pachyrhizi Sydow & P. Sydow, 1914
Taxonomía: Eukaryota > Fungi > Dikarya > Basidiomycota > Pucciniomycotina > Pucciniomycetes > Pucciniales > Phakopsoraceae > Phakopsora
.
.
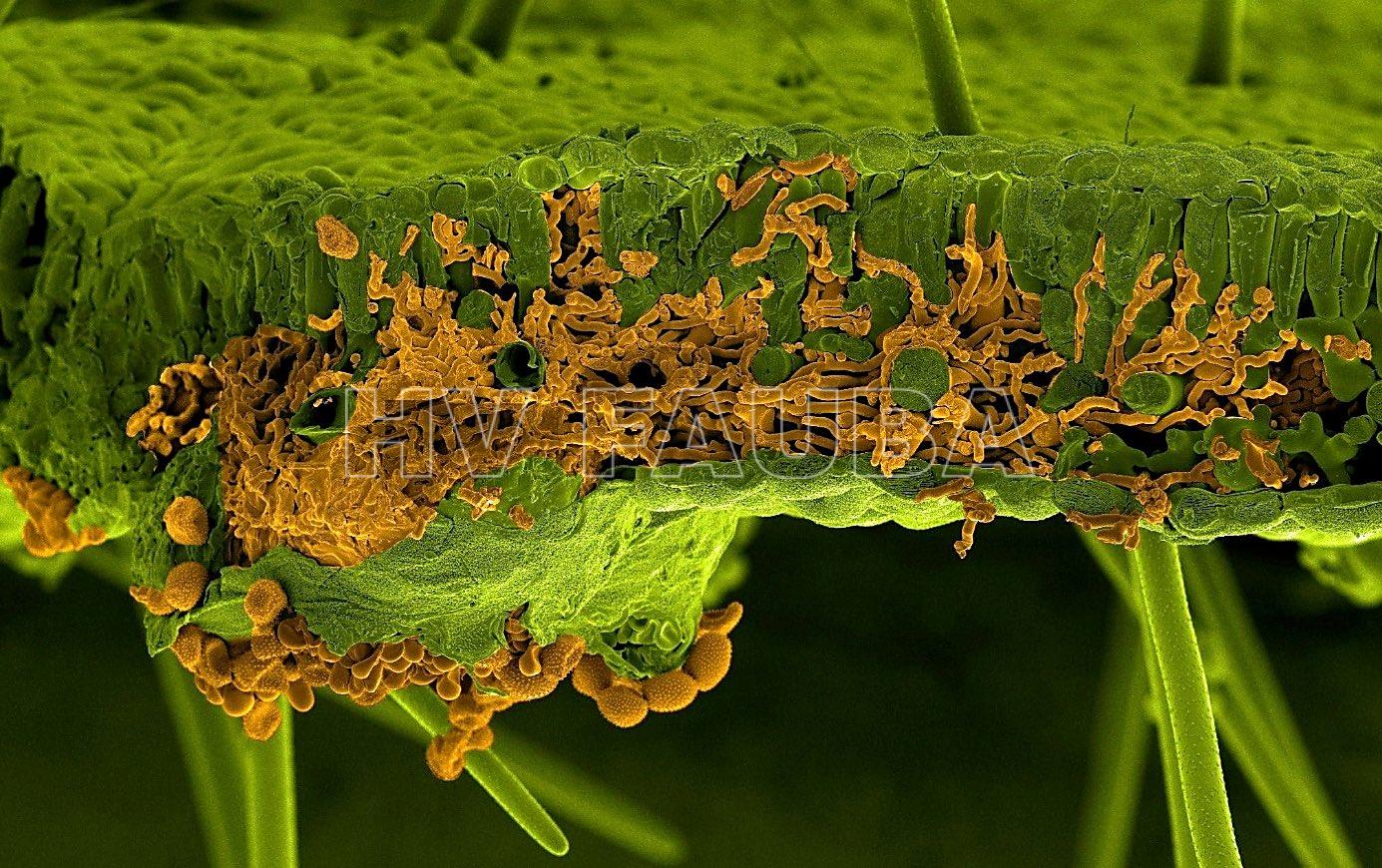
- Imagen de microscopía electrónica de barrido (SEM) de una hoja de soja infectada por Phakopsora pachyrhizi. La hoja y el hongo fueron pintados artificialmente de verde y naranja, respectivamente. La sección muestra hifas de infección invasora del hongo dentro del mesófilo de la hoja, mientras que las esporas son visibles debajo de la hoja que atraviesa la epidermis inferior (Autor: U. Steffens, Bayer Crop Science)
.
.
.
.
Antecedentes
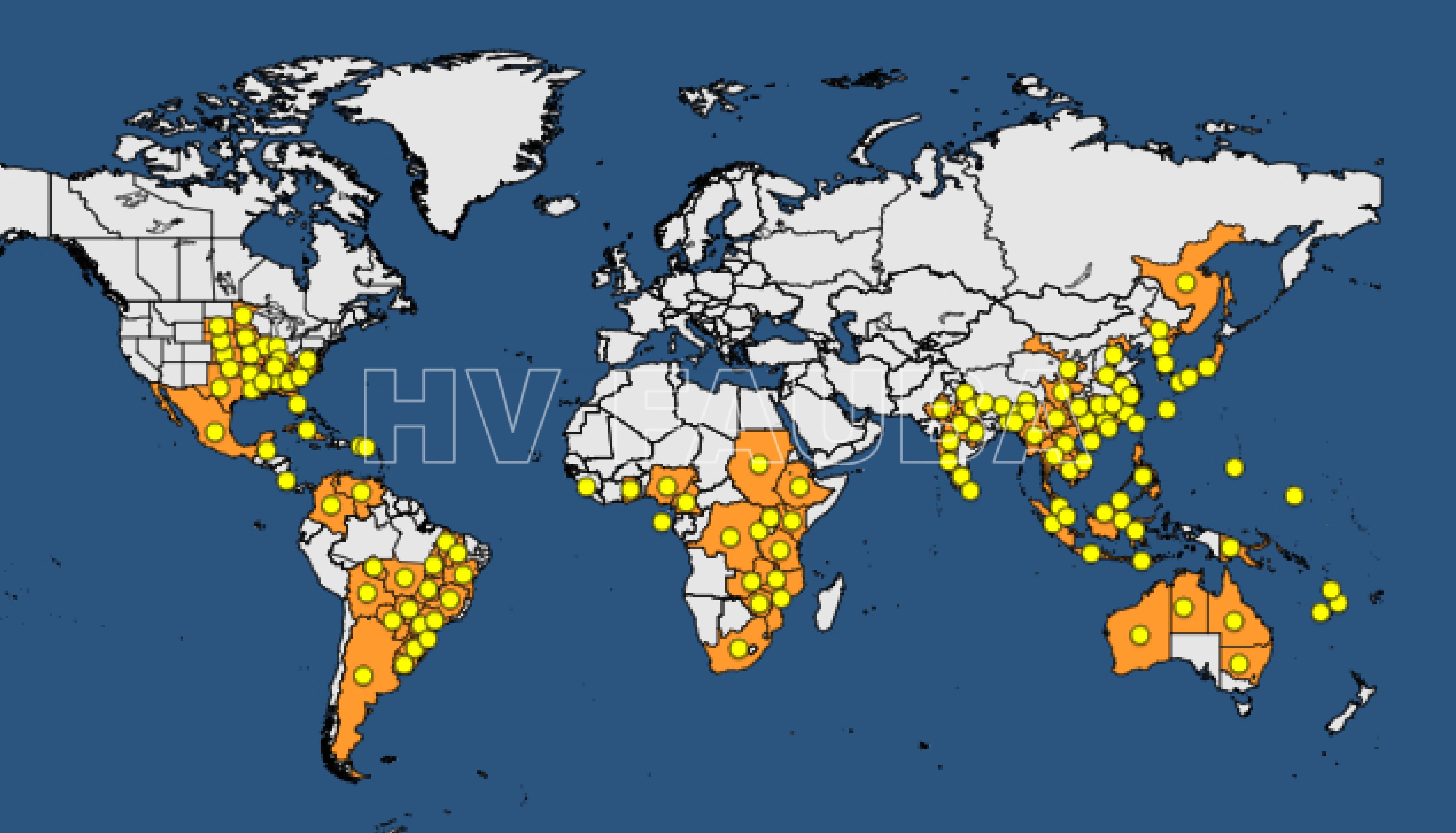
En América del Sur se encuentra en Paraguay, Brasil, Bolivia, Colombia, Uruguay, y en el NEA y NOA de la Argentina. Su importancia relativa, potencial muy elevada. Debido al tamaño pequeño (0,5 mm2) de las lesiones necróticas resulta muy difícil la detección a simple vista. La presencia de las pústulas es un indicio certero de la presencia de la enfermedad. Para la observación de sus pústulas se requiere una lupa de 20 aumentos o superior.
.
- Distribución mundial de la presencia de la roya de la soja
.
.
Síntomas y signos
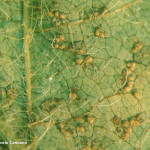
Las hojas son los órganos más frecuentemente afectados por esta enfermedad. Inicialmente, se manifiesta mediante diminutas lesiones o manchas puntiformes de color grisáceo a amarillento, ocultas en las hojas inferiores, fundamentalmente en el haz. Luego, estos puntos restringidos por las nervaduras, oscurecen adquiriendo una forma poligonal y angular. Se transforman en lesiones necróticas que son fácilmente confundibles con las causadas por otras enfermedades como mancha marrón, tizón y pústula bacteriana. La observación de estos síntomas es más frecuente en la parte basal del cultivo que en la superior. El amarilleo de las hojas inferiores (a veces de aspecto de “mosaico”), también es un síntoma clave a tener en cuenta para comenzar la observación. En condiciones óptimas para el desarrollo de la enfermedad, la sintomatología descripta es acompañada por una intensa defoliación que comienza por las hojas inferiores.
.
- Síntomas y signos de roya de la soja en el envéz de una hoja de soja.
.
Condiciones predisponentes para el establecimiento de la enfermedad
El período crítico se sitúa entre los 15 y 28 ºC, humedad relativa elevada (80%), y períodos de mojado foliar de 6 horas como mínimo.
.
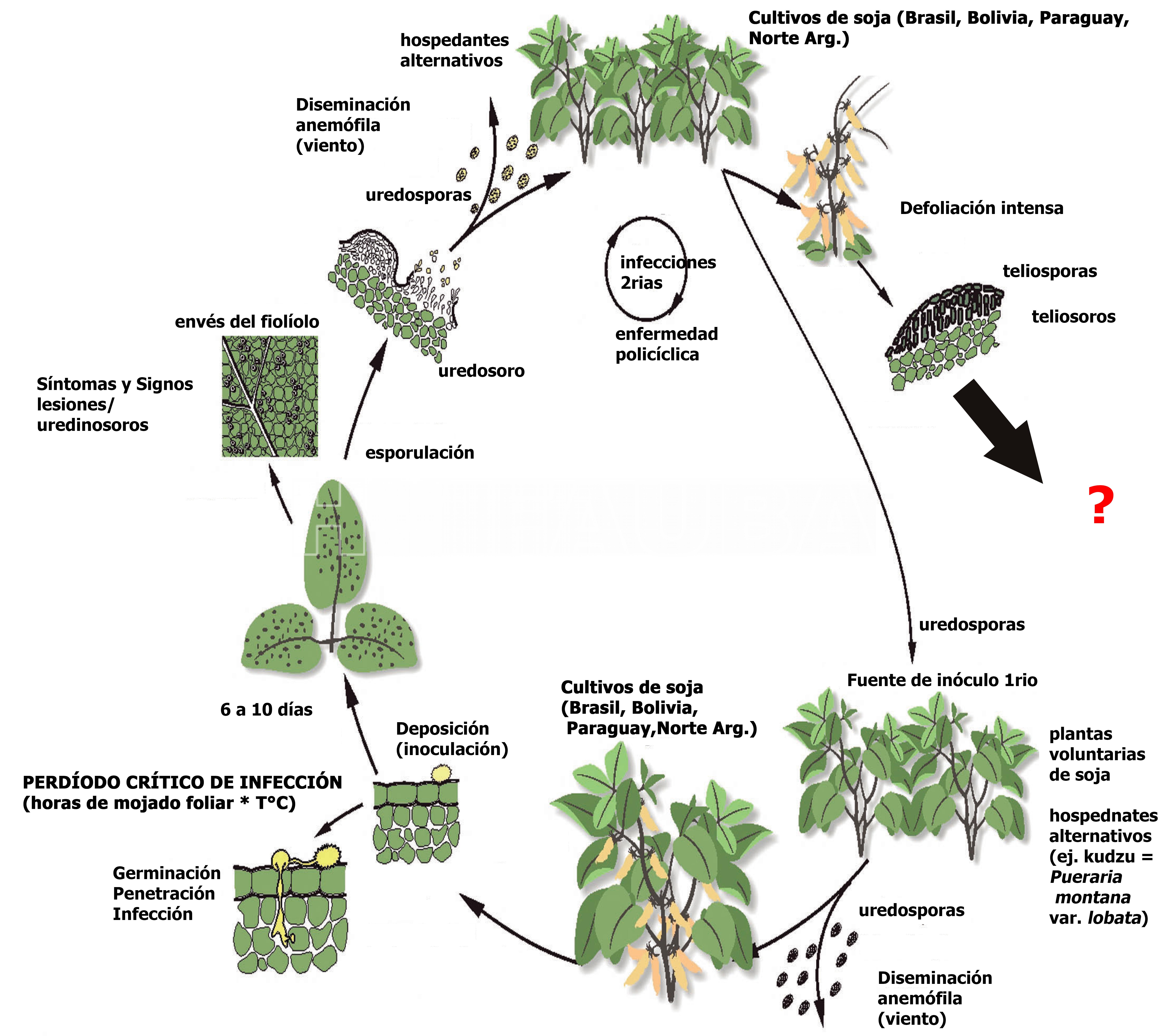
Ciclo de la enfermedad
- Ciclo biológico-agronómico de la roya de la soja, causada por Phakopsora pachyrhizi. Autor: Reis y Carmona, citado en Reis et al., 2006.
.
Epidemiología
Esta enfermedad no es una roya típica, ya que a diferencia de la mayoría de las royas (recordar por ej., las royas específicas de los cereales cuyas pústulas son anaranjadas o amarillas y no generan manchas), la RAS presenta lesiones necróticas, no genera fructificaciones de color vistoso, no es específica de la soja, puede causar intensa defoliación y en su comienzo es de difícil diagnóstico. Al igual que otras royas, la RAS, se disemina muy eficientemente por el viento (Belinelli et al., 2022) y no puede sobrevivir en semilla o rastrojo, por lo cual su manejo es muy diferente al de las EFC (enfermedades de fin de ciclo de la soja). En Argentina sobrevive en plantas voluntarias o guachas de soja y en el kudzu (Pueraria montana var. lobata (Willd.) Sanjappa & Pradeep), planta perenne de la familia botánica de la soja, que crece principalmente en Misiones.
Phakopsora pachyrizi ingresa a la hoja penetrando directamente en la epidermis de la planta y, en este caso, la hidrofobicidad o las señales de cera juegan un papel en la inducción de estructuras previas a la penetración, en lugar del tigmotropismo (Uppalapati et al., 2012; Ishiga et al., 2013).
.
- Ensayos de campo de fungicidas en Mato Grosso do Sul, Brasil, donde se observa parcela testigo sin tratar en primer plano, con plantas de soja que muestran un alto nivel de infección con Phakopsora pachyrhizi. Autor: Lamprecht, Bayer Division Crop Science.
.
Manejo Integrado
Actualmente no existen variedades de soja resistentes a la roya.
Es importante capacitarse en el reconocimiento y monitoreo de la enfermedad, eliminar plantas guachas. Las medidas preferenciales de manejo son: siembra de variedades de ciclos cortos, manejo de plantas guachas, monitoreo sistemático y control químico.
El monitoreo es la clave del manejo de esta enfermedad. Sugerencias para llevarlo a cabo eficientemente: recorrer el lote en diagonal y extraer 20 a 40 folíolos centrales de la mitad inferior de las plantas al menos 1 a 2 veces por semana desde la floración en adelante. Para ello detenerse de vez en cuando y extraer los folíolos hasta completar de 20 a 40. Comenzar por los lotes sembrados más temprano. Si dentro del lote existen zonas amarillentas, marrones o de mayor concentración de humedad comenzar por ese sector. Si se tiene una gran superficie o varios lotes para monitorear, pueden agruparse en un gran “lote semejante” siempre que sean de la misma variedad, conducidos con similares prácticas de manejo e implantados en lotes de similares tipos de suelo y la misma o similar fecha de siembra (en un rango de hasta 10 días).
.
.
- Pústulas de la Roya asiática de la soja (RAS), causada por Phakopsora pachyrhizi Sydow & Sydow; Roya asiática de la soja (RAS)
- Pústulas de la Roya asiática de la soja (RAS), causada por Phakopsora pachyrhizi Sydow & Sydow; Roya asiática de la soja (RAS)
- Esporulación (uredosoros con uredosporas) de Phakopsora pachyrhizi en hoja de soja.
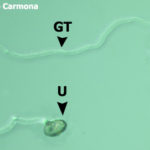
- Uredospora de Phakopsora pachyrhizi germinada (U = uredospora; GT = tubo germinativo; AP = apresorio)
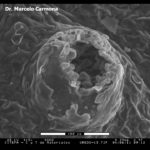
- Micrografía electrónica de barrido (SEM, scanning electron microscopy) de Uredosoro de Phakopsora pachyrhizi en hoja de soja
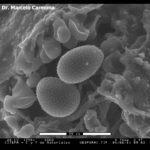
- Micrografía electrónica de barrido (SEM, scanning electron microscopy) de Uredosoro con uredosporas de Phakopsora pachyrhizi en hoja de soja.
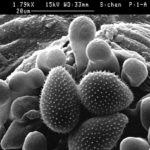
- Imagen de microscopía electrónica de barrido (SEM) de un uredio (pústula) y urediosporas de Phakopsora pachyrhizi, agebte causal de la roya asiática de la soja. Autor: Zhi-Yuan Chen.
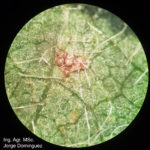
- 01 Pústula inicial de la Roya asiática de la soja (x16). Autor: Ing. Agr. MS. Jorge Dominguez.
- 02 Pústulas de la Roya asiática de la soja (x16). Autor: Ing. Agr. MS. Jorge Dominguez.
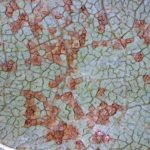
- 03 Pústulas de la Roya asiática de la soja (x16). Autor: Ing. Agr. MS. Jorge Dominguez..
- 04 Pústulas de la Roya asiática de la soja (x16). Autor: Ing. Agr. MS. Jorge Dominguez..
- 05 Pústulas (uredosoros) con esporulación (uredinosporas) de la Roya asiática de la soja. Autor: Ing. Agr. MS. Jorge Dominguez..
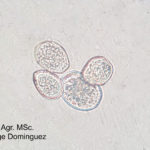
- 06 Observación microscópica de uredospora de Phakopsora pachyrhizi (x60) . Autor: Ing. Agr. MS. Jorge Dominguez
- Izquierda lote sin aplicación de fungicidas, con defoliación por RAS. Derecha, lote aplicado con fungicidas en R3. Autor: Ed Sikora
- Izquierda lote sin aplicación de fungicidas, con defoliación por RAS. Derecha, lote aplicado con fungicidas en R3. Autor: Ed Sikora
- Autor: Ed-Sikora
- Pústulas de Phakopsora pachyrhizi esporulando sobre hoja de Kudzu. Autor: Tom Allen
- Autor: Harun M. Murithi
- Autor: Harun M. Murithi
- Autor: Ed Sikora
- Autor: Ed Sikora
- 01 Uredinosoros de Phakopsora pachyrhizi en folíolos de soja. Autor: Dirceu Gassen
- 02 Uredinosoros de Phakopsora pachyrhizi en folíolos de soja. Autor: Dirceu Gassen
- 03 Uredinosoros de Phakopsora pachyrhizi en folíolos de soja. Autor: Dirceu Gassen
- 04 Síntomas de la roya de la soja, causados por Phakopsora pachyrhizi en folíolos de soja. Autor: Dirceu Gassen
- 05 Síntomas de la roya de la soja, causados por Phakopsora pachyrhizi en folíolos de soja. Autor: Dirceu Gassen
- 06 Uredinosoros de Phakopsora pachyrhizi en folíolos de soja. Autor: Dirceu Gassen
- 07 Uredinosoros de Phakopsora pachyrhizi en folíolos de soja. Autor: Dirceu Gassen
- 08 Uredinosoros de Phakopsora pachyrhizi en folíolos de soja. Autor: Dirceu Gassen
- 09 Uredinosoros de Phakopsora pachyrhizi en folíolos de soja. Autor: Dirceu Gassen
- 10 Uredinosoros de Phakopsora pachyrhizi en folíolos de soja. Autor: Dirceu Gassen
- 11 Uredinosoros de Phakopsora pachyrhizi en folíolos de soja. Autor: Dirceu Gassen
- 12 Síntomas de la roya de la soja, causados por Phakopsora pachyrhizi en folíolos de soja. Autor: Dirceu Gassen
.
.
.
Videos
Rust: the fungi that attacks plants. Created by Chris Hammang, Producer Sean O’Donoghue, Scientific Consultant Peter Dodds. C SIRO (Video)
.
Bibliografía
Akamatsu H, Yamanaka N, Yamaoka Y, et al. (2013) Pathogenic diversity of soybean rust in Argentina, Brazil, and Paraguay. Journal of General Plant Pathology 79: 28–40. doi: 10.1007/s10327-012-0421-7
Alves KS, Barro JP, Guimarães M, Del Ponte EM (2021) Profitability of fungicide applications for managing soybean rust in scenarios of variable efficacy and costs: a stochastic simulation. Plant Pathol. Accepted Author Manuscript. doi: 10.1111/ppa.13396
Arsenault-Labrecque G, Menzies JG, Bélanger RR (2012) Effect of Silicon Absorption on Soybean Resistance to Phakopsora pachyrhizi in Different Cultivars. Plant Dis. 96(1): 37-42. doi: 10.1094/PDIS-05-11-0376
Barro P, Alves KS, Godoy CV (2021) Performance of dual and triple fungicide premixes for managing soybean rust across years and regions in Brazil: a meta-analysis. Plant Pathology. Accepted Author Manuscript. doi: 10.1111/ppa.13418
Belinelli EO, Fantin LH, Canteri MG, et al. (2022) Numerical simulation of atmospheric transport of Phakopsora pachyrhizi urediniospores in South America using the state of Paraná-Brazil as a model. Eur J Plant Pathol 163: 979–990. doi: 10.1007/s10658-022-02533-7
Beyer SF, Bel PS, Flors V, et al. (2021) Disclosure of salicylic acid and jasmonic acid-responsive genes provides a molecular tool for deciphering stress responses in soybean. Sci Rep 11: 20600. doi: 10.1038/s41598-021-00209-6
Bonde MR, Nester SE, Moore WF, Allen TW (2009) Comparative susceptibility of kudzu accessions from the southeastern United States to infection by Phakopsora pachyrhizi. Plant Disease 93: 593-598. doi: 10.1094/PDIS-93-6-0593
Bruzamarello J, Franceschi VT, Da Cruz MP, et al. (2018) Defense activation in soybean by the action of amino acids and micronutrient against Phakopsora pachyrhizi. Australian Journal of Basic and Applied Sciences 12(2): 6–9. doi: 10.22587/ajbas.2018.12.2.2
Bruzamarello J, Franceschi VT, Locatelli Dalacosta N, et al. (2018) Potencial de fosfitos na indução da resistência em plantas de soja. Cultura Agronômica 27. doi: 10.32929/2446-8355.2018v27n3p263-273
Carmona MA, Gally ME, Lopez SE (2005) Asian Soybean Rust: Incidence, Severity, and Morphological Characterization of Phakopsora pachyrhizi (Uredinia and Telia) in Argentina. Plant Disease 89: 109-109. doi: 10.1094/PD-89-0109B
Carmona M, Gally M, López S (2007) Studies on of Asian Soybean Rust (Phakopsora pachyrhizi) in Argentina. Fitopatología 42: 25-30.
Chang HX, Miller LA, Hartman GL (2014) Melanin-independent accumulation of turgor pressure in appressoria of Phakopsora pachyrhizi. Phytopathology 104(9): 977–984. doi: 10.1094/PHYTO-12-13-0335-R
Chicowski AS, Bredow M, Utiyama AS, et al. (2024) Soybean-Phakopsora pachyrhizi interactions: towards the development of next-generation disease-resistant plants. Plant Biotechnol. J, 22: 296-315. doi: 10.1111/pbi.14206
Claus A, Simões K, De Mio LLM (2022) SdhC-I86F Mutation in Phakopsora pachyrhizi Is Stable and Can Be Related to Fitness Penalties. Phytopathology 112(7): 1413-1421. doi: 10.1094/PHYTO-10-21-0419-R
Claus A, Roncatto E, Barroso AAM, May De Mio LL (2023) Herbicides reduce the severity and sporulation of Phakopsora pachyrhizi in soybean with triple herbicide resistance. Pest Manag Sci. doi: 10.1002/ps.7557
Cruz MFA, Rodrigues FÁ, Diniz APC, et al. (2014) Soybean Resistance to Phakopsora pachyrhizi as Affected by Acibenzolar‐S‐Methyl, Jasmonic Acid and Silicon. J Phytopathol, 162: 133-136. doi: 10.1111/jph.12170
Dalla Lana F, Paul PA, Godoy CV, et al. (2018) Meta-Analytic Modeling of the Decline in Performance of Fungicides for Managing Soybean Rust after a Decade of Use in Brazil. Plant Disease 102(4): 807-817. doi: 10.1094/PDIS-03-17-0408-RE
de Carvalho MC, Costa Nascimento L, Darben LM, et al. (2016) Prediction of the in planta Phakopsora pachyrhizi secretome and potential effector families. Molecular Plant Pathology 18(3): 363–377. doi: 10.1111/mpp.12405
de Mello FE, Mathioni SM, Fantin LH, et al. (2021) Sensitivity assessment and SDHC‐I86F mutation frequency of Phakopsora pachyrhizi populations to benzovindiflupyr and fluxapyroxad fungicides from 2015 to 2019 in Brazil. Pest Management Science Accepted Author Manuscript. doi: 10.1002/ps.6466
de Paula S, Holz S, Garcia Souza DH, Pascholat SF (2021) Potential of resistance inducers for soybean rust management. Canadian Journal of Plant Pathology 43: S298-S307. doi: 10.1080/07060661.2021.1977999
de Souza DM, Raetano CG, Negrisoli MM, et al. (2022) Impact of fungicide application volume on ASIAN rust control and soybean crop yield. Eur J Plant Pathol . doi: 10.1007/s10658-022-02524-8
Del Ponte EM, Godoy CV, Li X, Yang XB (2006) Predicting Severity of Asian Soybean Rust Epidemics with Empirical Rainfall Models. Phytopathology 96(7): 797-803. doi: 10.1094/PHYTO-96-0797
Del Ponte EM, Godoy CV, Canteri MG, et al. (2006) Models and applications for risk assessment and prediction of Asian soybean rust epidemics. Fitopatologia Brasileira 31: 533-544. doi: 10.1590/S0100-41582006000600001
du Preez ED, van Rij NC, Lawrance KF, et al. (2005) First Report of Soybean Rust Caused by Phakopsora pachyrhizi on Dry Beans in South Africa. Plant Disease 89(2): 206. doi: 10.1094/PD-89-0206C
Edwards HH, Bonde MR (2011) Penetration and establishment of Phakopsora pachyrhizi in soybean leaves as observed by transmission electron microscopy. Phytopathology 101(7): 894-900. doi: 10.1094/PHYTO-09-10-0248
Enciso-Maldonado GA, Maidana-Ojeda M, Schlickmann-Tank JA, et al. (2019) Fungicidas sitio-específicoscombinados con Mancozeb para el control de la roya asiática de la soya. Revista Mexicana de Fitopatología37 (No. Esp. 1) 15-21. doi: 10.18781/R.MEX.FIT.1903-3
Fernández-Salinas P, Enciso-Maldonado GA (2019) Potassium phosphite and chitosan on the control of asian soybean rust. XXI Congreso Internacional y XLVI Congreso Nacional de la Sociedad Mexicana de Fitopatología. Agosto 2019. Link
Ferreira EGC, Marcelino-Guimarães FC (2022) Mapping Major DiseaseResistance Genes in Soybean by Genome-Wide Association Studies. In: Torkamaneh D., Belzile F. (eds) Genome-Wide Association Studies. Methods in Molecular Biology, vol 2481. Humana, New York, NY. doi: 10.1007/978-1-0716-2237-7_18
, , , A new standard area diagram set for assessment of severity of soybean rust improves accuracy of estimates and optimizes resource use. Plant Pathology 69: 495– 505. doi: 10.1111/ppa.13148
Garcia A, Calvo ES, de Souza Kiihl RA, et al. (2008) Molecular mapping of soybean rust (Phakopsora pachyrhizi) resistance genes: discovery of a novel locus and alleles. Theor Appl Genet. 117(4): 545-53. doi: 10.1007/s00122-008-0798-z
Gill US, Sun L, Rustgi S, et al. (2018) Transcriptome based analyses of phosphite mediated suppression of rust pathogens, Puccinia emaculata and Phakopsora pachyrhizi, and functional characterization of selected fungal target genes. The Plant Journal (Accepted Manuscript). doi: 10.1111/tpj.13817
Godoy CV, Santos Seixas CD, Moreira Soares R, et al. (2016) Asian soybean rust in Brazil: past, present, and future. Pesquisa Agropecuária Brasileira 51(5): 407-421. doi: 10.1590/S0100-204X2016000500002
Goellner K, Loehrer M, Langenbach C, et al. (2010) Phakopsora pachyrhizi, the causal agent of Asian soybean rust. Molecular Plant Pathology 11(2): 169-177. doi: 10.1111/j.1364-3703.2009.00589.x
Gomes Barros L, Barbosa Avelino B, da Silva DCG, et al. (2022) Mapping of a soybean rust resistance in PI 594756 at the Rpp1 locus. PREPRINT. Research Square. doi: 10.21203/rs.3.rs-1918508/v1
Gupta YK, Marcelino-Guimarães FC, Lorrain C, et al. (2023) Major proliferation of transposable elements shaped the genome of the soybean rust pathogen Phakopsora pachyrhizi. Nat Commun 14: 1835. doi: 10.1038/s41467-023-37551-4
, , , (2022) A major variation in the virulence of the Asian soybean rust pathogen (Phakopsora pachyrhizi) in Bangladesh. Plant Pathology 71: 1355– 1368. doi: 10.1111/ppa.13568
Ishiga Y, Upplapapti SR, Mysore KS (2013) Expression analysis reveals a role for hydrophobic or epicuticular wax signals in pre-penetration structure formation of Phakopsora pachyrhizi. Plant Signal. Behav. 8: e26959. doi: 10.4161/psb.26959
Jorge VR, Silva MR, Guillin EA, et al. (2015) The origin and genetic diversity of the causal agent of Asian soybean rust, Phakopsora pachyrhizi, in South America. Plant Pathology 64(3): 729–737. doi: 10.1111/ppa.12300
Kashiwa T, Muraki Y, Yamanaka N (2020) Near-isogenic soybean lines carrying Asian soybean rust resistance genes for practical pathogenicity validation. Scientific Reports 10: 13270. doi: 10.1038/s41598-020-70188-7
Kato M (2017) Effectiveness of Resistance Genes to the Soybean Rust Pathogen Phakopsora pachyrhizi. Japan Agricultural Research Quarterly 51: 199-207. doi: 10.6090/jarq.51.199
Kato M, Soares RM (2022) Field trials of a Rpp-pyramided line confirm the synergistic effect of multiple gene resistance to Asian soybean rust (Phakopsora pachyrhizi). Trop. plant pathol. 47: 222–232. doi: 10.1007/s40858-021-00471-z
Kato M, Morel A, Yamanaka N (2022) Resistance to Asian soybean rust and yield of new soybean cultivars, JFNC 1 and JFNC 2, harboring three resistance genes. Trop. plant pathol. 47: 599–607. doi: 10.1007/s40858-022-00516-x
Kelly HY, Dufault NS, Walker DR, et al. (2015) From Select Agent to an Established Pathogen: The Response to Phakopsora pachyrhizi (Soybean Rust) in North America. Phytopathology 105(7): 905-916. doi: 10.1094/PHYTO-02-15-0054-FI
Klosowski AC, Castellar C, Stammler G, May De Mio LL (2018) Fungicide sensitivity and monocyclic parameters related to the Phakopsora pachyrhizi–soybean pathosystem from organic and conventional soybean production systems. Plant Pathology. doi:10.1111/ppa.12883
Langenbach C, Campe R, Beyer SF, et al. (2016) Fighting Asian Soybean Rust. Frontiers in Plant Science 7: 797. doi: 10.3389/fpls.2016.00797
Larzábal J, Yamanaka N, Ceretta S, et al. (2022) Introgression of Asian soybean rust resistant genes into elite soybean lines from Uruguay. International Journal of Pest Management 68: 319-327. doi: 10.1080/09670874.2022.2118894
Li X, Esker PD, Pan Z, et al. (2010) The Uniqueness of the Soybean Rust Pathosystem: An Improved Understanding of the Risk in Different Regions of the World. Plant Disease 94(7): 796-806. doi: 10.1094/PDIS-94-7-0796
Lin F, Chhapekar SS, Vieira CC, et al. (2022) Breeding for disease resistance in soybean: a global perspective. Theor Appl Genet 135: 3773–3872. doi: 10.1007/s00122-022-04101-3
Lintz J, Dubrulle G, Cawston E, et al. (2022) A Short Review of Anti-Rust Fungi Peptides: Diversity and Bioassays. Front. Agron. 4: 966211. doi: 10.3389/fagro.2022.966211
Loehrer M, Vogel A, Huettel B, et al. (2014) On the current status of Phakopsora pachyrhizi genome sequencing. Frontiers in Plant Science 5: 377. doi: 10.3389/fpls.2014.00377
Machado FJ, Barro JP, Godoy CV, et al. (2022) Is tank mixing site-specific premixes and multi-site fungicides effective and economic for managing soybean rust? a meta-analysis. Crop Protection 151: 105839. doi: 10.1016/j.cropro.2021.105839
, , , et al. (2021) Evaluation of soybean genotypes for resistance against the rust‐causing fungus Phakopsora pachyrhizi in East Africa. Plant Pathology 00: 1– 12. doi: 10.1111/ppa.13339
Meyer JDF, Silva DCG, Yang C, et al. (2009) Identification and Analyses of Candidate Genes for Rpp4-Mediated Resistance to Asian Soybean Rust in Soybean. Plant Physiology 150(1): 295–307. doi: 10.1104/pp.108.134551
Müller A, Stammler G, May De Mio LL (2021) Multiple resistance to DMI, QoI and SDHI fungicides in field isolates of Phakopsora pachyrhizi. Crop Protection 105618. doi: 10.1016/j.cropro.2021.105618
Murithi HM, Beed F, Tukamuhabwa P, et al. (2015) Soybean production in eastern and southern Africa and threat of yield loss due to soybean rust caused by Phakopsora pachyrhizi. Plant Pathology 65(2): 176–188. doi: 10.1111/ppa.12457
, , , et al. Diversity and distribution of pathotypes of the soybean rust fungus Phakopsora pachyrhizi in East Africa. Plant Pathology 00: 1– 12. doi: 10.1111/ppa.13324
Narváez DF, Jurick WM II, Marois JJ, Wright DL (2010) Effects of surface wetness periods on development of soybean rust under field conditions. Plant Disease 94: 258-264. doi: 10.1094/PDIS-94-2-0258
Nolla A, Korndörfer GH, Coelho L (2006) Efficiency of Calcium Silicate and Carbonate in Soybean Disease Control. Journal of Plant Nutrition 29: 2049-2061. doi: 10.1080/01904160600932658
Ono Y, Buriticá P, Hennen JF (1992) Delimitation of Phakopsora, Physopella and Cerotelium and their species on Leguminosae. Mycological Research 96: 825-850. doi: 10.1016/S0953-7562(09)81029-0
Picanço BBM, Silva BN, Rodrigues FA (2022) Potentiation of soybean resistance against Phakopsora pachyrhizi infection using phosphite combined with free amino acids. Plant Pathology 71: 1496– 1510. doi: 10.1111/ppa.13582
(2023) Soybean and grapevine rusts accelerate the defoliation rates of host plants. Plant Pathology 00: 1–9. doi: 10.1111/ppa.13841
Qi M, Link TI, Müller M, et al. (2016) A Small Cysteine-Rich Protein from the Asian Soybean Rust Fungus, Phakopsora pachyrhizi, Suppresses Plant Immunity. PLoS Pathog 12(9): e1005827. doi: 10.1371/journal.ppat.1005827
Radons SZ, Heldwein AB, Puhl AJ, et al. (2021) Climate risk of Asian soybean rust occurrence in the state of Rio Grande do Sul, Brazil. Trop. plant pathol. 46: 435–442. doi: 10.1007/s40858-021-00431-7
Reis EM, Bresolin ACR, Carmona M (2006) Doenças da soja I: Ferrugem asiática. Universidade de Passo Fundo, Passo Fundo.
Reis EM, Zanatta M, Moreira EN, et al. (2006) Curva de progresso da ferrugem da soja em Passo Fundo-RS. Fitopatologia Brasileira 31 (Suplemento): S139 (Resumo)
Reis EM, Zanatta M, Reis AC (2021) Addition of Mancozeb to DMI + QoI, and SDHI + QoI Co-formulations Improving Control of Asian Soybean Rust. Journal of Agricultural Science 13: 195-201. Link
Reis EM, Dias Guerra W, Zambolim L, et al. (2022) Minimizing the Selection Pressure of Site-Specific Fungicides Towards Phakopsora pachyrhizi in Mato Grosso State: A Review. Journal of Agricultural Science 14(5): 134-141. doi: 10.5539/jas.v14n5p134
Reis EM, et al. (2022) Sobre os danos causados pela ferrugem asiática da soja. Summa Phytopathologica 48: 53-54. doi: 10.1590/0100-5405/230240
Reznikov S, De Lisi V, Claps P, et al. (2019) Evaluation of the efficacy and application timing of different fungicides for management of soybean foliar diseases in northwestern Argentina. Crop Protection 124: 104844. doi: 10.1016/j.cropro.2019.104844
Rincão MP, Carvalho MCCG, Nascimento LC, et al. (2018) New insights into Phakopsora pachyrhizi infection based on transcriptome analysis in planta. Genetics and Molecular Biology 41: 671-691. doi: 10.1590/1678-4685-gmb-2017-0161
Rossi RL (2003) First Report of Phakopsora pachyrhizi, the Causal Organism of Soybean Rust in the Province of Misiones, Argentina. Plant Disease 87(1): 102-102. doi: 10.1094/PDIS.2003.87.1.102A
Santana Torres Y, Martínez de la Parte E, Pérez Vicente L, et al. (2012) Hospedantes alternativos de Phakopsora pachyrhizi en campos de soya (Glycine max) de la provincia de Ciego de Ávila, Cuba Fitosanidad 16: 69-72. Link
Schmitz HK, Medeiros C-A, Craig IR, Stammler G (2014) Sensitivity of Phakopsora pachyrhizi towards quinone-outside-inhibitors and demethylation-inhibitors, and corresponding resistance mechanisms. Pest. Manag. Sci., 70: 378-388. doi: 10.1002/ps.3562
Schneider KT, van de Mortel M, Bancroft TJ, et al. (2011) Biphasic Gene Expression Changes Elicited by Phakopsora pachyrhizi in Soybean Correlate with Fungal Penetration and Haustoria Formation. Plant Physiology 157: 355–371. doi: 10.1104/pp.111.181149
Sikora EJ, Allen TW, Wise KA, et al. (2014) A Coordinated Effort to Manage Soybean Rust in North America: A Success Story in Soybean Disease Monitoring. Plant Disease 98(7): 864-875. doi: 10.1094/ PDIS-02-14-0121-FE
Silva E, da Graça JP, Porto C, et al. (2020) Unraveling Asian Soybean Rust metabolomics using mass spectrometry and Molecular Networking approach. Scientific Reports 10(1): 138. doi: 10.1038/s41598-019-56782-4
Simões K, Hawlik A, Rehfus A, et al. (2018) First detection of a SDH variant with reduced SDHI sensitivity in Phakopsora pachyrhizi. J Plant Dis Prot 125: 21–26. doi: 10.1007/s41348-017-0117-5
Simony Ribeiro A, Ferraz de Toledo JF, Arrabal Arias CA, et al. (2008) Genetic control of soybean (Glycine max) yield in the absence and presence of the Asian rust fungus (Phakopsora pachyrhizi). Genetics and Molecular Biology 31(1): 98-105. doi: 10.1590/S1415-47572008000100018
Slaminko TL, Miles MR, Frederick RD, et al. (2008) New legume hosts of Phakopsora pachyrhizi based on greenhouse evaluations. Plant Disease 92: 767–771. doi: 10.1094/PDIS-92-5-0767
Soto N, Hernández Y, Delgado C, et al. (2020) Field Resistance to Phakopsora pachyrhizi and Colletotrichum truncatum of Transgenic Soybean Expressing the NmDef02 Plant Defensin Gene. Frontiers in Plant Science 11: 562. doi: 10.3389/fpls.2020.00562
Stone CL, McMahon MB, Fortis LL, et al. (2012) Gene expression and proteomic analysis of the formation of Phakopsora pachyrhizi appressoria. BMC Genomics 22;13: 269. doi: 10.1186/1471-2164-13-269
Tremblay A (2011) Soybean Rust: Five Years of Research. In: Sudaric A (Ed.), Soybean – Molecular Aspects of Breeding. InTech, ISBN: 978-953-307-240-1. doi: 10.5772/15121
Twizeyimana M, Hartman GL (2010) Culturing Phakopsora pachyrhizi on detached leaves and urediniospore survival at different temperatures and relative humidities. Plant Disease 94: 1453-1460. doi: 10.1094/PDIS-02-10-0131
Twizeyimana M, Hartman GL (2017) Sensitivity of Phakopsora pachyrhizi Isolates to Fungicides and Reduction of Fungal Infection Based on Fungicide and Timing of Application. Plant Disease 101(1): 121-128. doi: 10.1094/PDIS-04-16-0552-RE
Twizeyimana M, Hammer PE, Gachango E, et al. (2023) Diverse environmental bacteria displaying activity against Phakopsora pachyrhizi, the cause of soybean rust. Front. Plant Sci. 14: 1080116. doi: 10.3389/fpls.2023.1080116
Uppalapati SR, Ishiga Y, Doraiswamy V, et al. (2012) Loss of abaxial leaf epicuticular wax in Medicago truncatula irg1/palm1 mutants results in reduced spore differentiation of anthracnose and nonhost rust pathogens. Plant Cell 24: 353–370. doi: 10.1105/tpc.111.093104
Wen L, Bowen CR, Hartman GL (2017) Prediction of short-distance aerial movement of Phakopsora pachyrhizi urediniospores using machine learning. Phytopathology 107(10): 1187-1198. doi: 10.1094/PHYTO-04-17-0138-FI
Yamanaka N, Giacomini Lemos N, Chávez Jara R, et al. (2015) Prevention of leaf yellowing in Asian soybean rust infected plants is associated with green cotyledon color and the infection index. Euphytica 205(2): 475-482. doi: 10.1007/s10681-015-1414-3
Yorinori JT, Paiva WM, Frederick RD, et al. (2005) Epidemics of soybean rust (Phakopsora pachyrhizi) in Brazil and Paraguay from 2001 to 2003. Plant Disease 89(6): 675-677. doi: 10.1094/PD-89-0675
Zambolim L, Reis EM, Guerra WD, et al. (2022). Integrated Management of Asian Soybean Rust. European Journal of Applied Sciences 10(2): 602–633. doi: 10.14738/aivp.102.12215