.
Condición fitosanitaria: Plaga no cuarentenaria reglamentada
Grupo de cultivos: Oleaginosas
Rango de hospedantes: Ampio, no específico, Cientos a miles de especies, incluyendo cutlivos extensivos de grano, pastos, hortalizas, frutales y numerosas malezas.
Especie hospedante: Soja (Glycine max)
.
Agente causal:
Meloidogyne javanica (Treub, 1885),
M. incognita (Kofoid & White, 1919)(southern root knot),
M. hapla Chitwood, 1949 (northern root knot),
M. arenaria (Neal, 1889)(peanut root knot),
M. enterlobii (guava root knot),
Meloidogyne spp. (~100 species)
.
Taxonomía: Eukaryota > Opisthokonta > Metazoa > Eumetazoa > Bilateria > Protostomia > Ecdysozoa > Nematoda > Chromadorea > Rhabditida > Tylenchina > Tylenchomorpha > Tylenchoidea > Meloidogynidae > Meloidogyninae > Meloidogyne > Meloidogyne incognita group
.
.
.
Síntomas

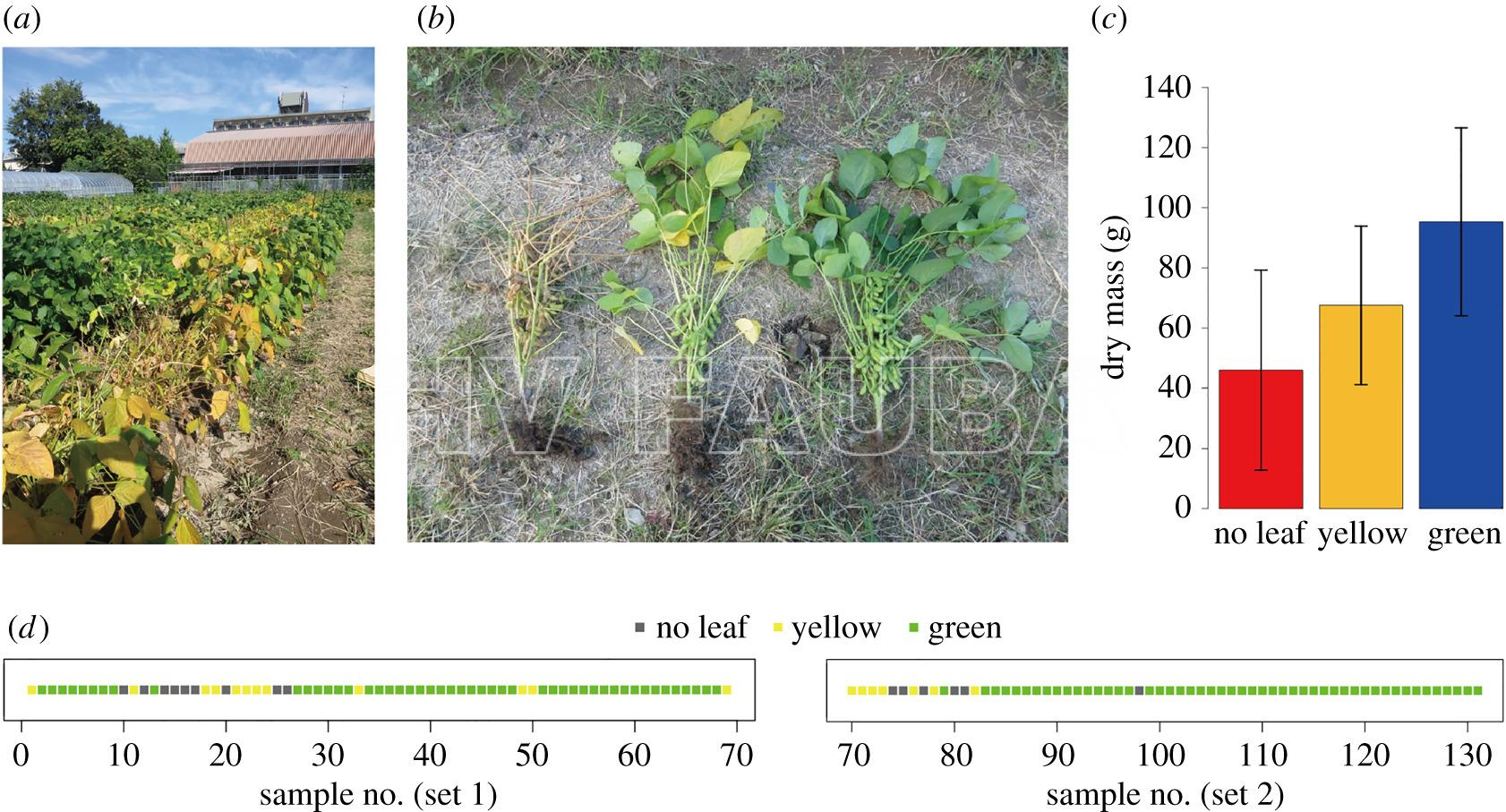
Síntomas foliares del “nematodo del nudo de la raíz” o “nematodo de la agalla” (root-knot nematode) en la soja que incluyen amarillamiento y muerte de las hojas. Autor: T. Faske.

Agallas en las raíces de la soja causadas por la infección del “nematodo del nudo de la raíz” o “nematodo de la agalla” (root-knot nematode). Autor: T. Faske.
.
- Autor: Toju y Tanaka, 2019.
.
- Autor: Tom Allen
- Autor: Tom Allen
- Autor: Tom Allen
- Autor: Eros Francsico
- Autor: Tom Allen
- Autor: Tom Allen
- Autor: Tom Allen
.
.
.
Bibliografía
Crop Protection Network. Soybean Cyst Nematode of Soybean
video Root-knot nematode and soybean variety tolerance. Auburn University
Fighting the ‘Invasion of the Body Snatchers’ in Soybeans
Alekcevetch JC, de Lima Passianotto AL, Ferreira EGC, et al. (2021) Genome-wide association study for resistance to the Meloidogyne javanica causing root-knot nematode in soybean. Theor Appl Genet 134: 777–792. doi: 10.1007/s00122-020-03723-9
, , , et al. (2022) Genetic variability and phylogenetic characterization of different populations of Meloidogyne izalcoensis and reaction of coffee genotypes to this new species detected in Brazil. Plant Pathology 00: 1– 15. doi: 10.1111/ppa.13686
Basnet P, Meinhardt CG, Dhital B, et al. (2024) Development of a Standardized Soybean Cyst Nematode Screening Assay in Pennycress and Identification of Resistant Germplasm. Plant Dis. 108(2): 359-364. doi: 10.1094/PDIS-05-23-0858-RE
Basso MF, Lourenço-Tessutti IT, Moreira-Pinto CE, et al. (2022) Overexpression of a soybean Globin (GmGlb1-1) gene reduces plant susceptibility to Meloidogyne incognita. Planta 256: 83. doi: 10.1007/s00425-022-03992-2
Basso MF, Lourenço-Tessutti IT, Moreira-Pinto CE, et al. (2022) Overexpression of the GmEXPA1 gene reduces plant susceptibility to Meloidogyne incognita. Plant Cell Rep. doi: 10.1007/s00299-022-02941-3
Bissonnette KM, Barizon J, Adee EA, et al. (2024) Management of soybean cyst nematode and sudden death syndrome with nematode-protectant seed treatments across multiple environments in soybean. Plant Disease. doi: 10.1094/PDIS-02-23-0292-RE
Carraro-Lemes CF, Deuner CC, Arruda KMA, et al. (2022) Host reaction of wheat genotypes to Meloidogyne javanica and M. incognita. Trop. plant pathol. 47: 770–775. doi: 10.1007/s40858-022-00529-6
Dağlı D, Duman N, Yüksel E, et al. (2023) Characterization of cereal cyst nematodes in wheat using morphometrics, SCAR markers, RFLP, and rDNA-ITS sequence analyses. Trop. plant pathol. 48: 207–216. doi: 10.1007/s40858-022-00528-7
Devaraja KP, Ellur RK, Pankaj, et al. (2022) Response of rice genotypes to rice root-knot nematode (Meloidogyne graminicola) infection under varying temperature regimes. Plant Pathology 00: 1– 16. doi: 10.1111/ppa.13647
Doucet ME (1993) Consideraciones acerca del género Meloidogyne Goeldi, 1987 (Nemata: Tylenchida) y su situación en Argentina. Asociaciones y distribución. Agriscientia X: 63-80.
Faske TR, Mueller J, Becker JO, et al. (2023) Summarized Distribution of the Southern Root-Knot Nematode, Meloidogyne incognita, in Field Crops in the United States. Plant Health Progress. doi: 10.1094/PHP-04-23-0031-BR
Figueiredo J, Vieira P, Abrantes I, Esteves I (2021) Detection of the root lesion nematode Pratylenchus penetrans in potato tubers. Plant Pathology 00: 1– 9. doi: 10.1111/ppa.13425
Forge T, Munro P, Midwood AJ, et al. (2021) Shifting Prevalence of Plant-Parasitic Nematodes in Orchards and Vineyards of the Okanagan Valley, British Columbia. Plant Health Progress 22: 113-121. doi: 10.1094/PHP-10-20-0079-RS
France RA, Abawi GS (1993) Interaction between Meloidogyne incognita and Fusarium oxysporum f. sp. phaseoli on selected bean genotypes. Journal of Nematology 26: 467-474.
, , , (2022) Registration of conventional soybean germplasm JTN-5110 with resistance to nematodes and fungal pathogens. Journal of Plant Registrations 00: 1– 13. doi: 10.1002/plr2.20254
Gheysen G, Mitchum MG (2019) Phytoparasitic Nematode Control of Plant Hormone Pathways. Plant Physiol. 179(4): 1212-1226. doi: 10.1104/pp.18.01067
Gorny AM, Hay FS, Esker P, Pethybridge SJ (2020) Spatial and spatiotemporal analysis of Meloidogyne hapla and Pratylenchus penetrans populations in commercial potato fields in New York, USA. Nematology 23(2)> 139-151. doi: 10.1163/15685411-bja10034
Gorny AM, Ye W, Cude S, Thiessen L (2021) Soybean Root-Knot Nematode: A Diagnostic Guide. Plant Health Progress 22: 164-175. doi: 10.1094/PHP-01-21-0005-DG
Goverse A, Mitchum MG (2022) At the molecular plant–nematode interface: New players and emerging paradigms. Current Opinion in Plant Biology 67: 102225. doi: 10.1016/j.pbi.2022.102225
Holderbaum MM, Santiago DC, Sera GH, et al. (2021) Penetration, development, and reproduction of Meloidogyne paranaensis in three Coffea arabica genotypes. Trop. plant pathol. 46: 528–535. doi: 10.1007/s40858-021-00449-x
Jain A, Wainer J, Huston DC, et al. (2022) Correction to: First report of a cyst nematode, Heterodera daverti, from Australia. Australasian Plant Dis. Notes 17: 39. doi: 10.1007/s13314-022-00485-9
Jain A, Huston DC, Wainer J, et al. (2023) Geographic range extension of hop cyst nematode, Heterodera humuli, from Tasmania to the Australian mainland. Australasian Plant Dis. Notes 18: 8. doi: 10.1007/s13314-023-00494-2
Kessler AC, Koehler AM (2023) Seed Treatments for Management of Soybean Cyst Nematode, Heterodera glycines, in Mid-Atlantic Soybean Production. J Nematol. 55(1): 20230026. doi: 10.2478/jofnem-2023-0026
Khan M, Khan AU, Bogdanchikova N, Garibo D (2021) Antibacterial and Antifungal Studies of Biosynthesized Silver Nanoparticles against Plant Parasitic Nematode Meloidogyne incognita, Plant Pathogens Ralstonia solanacearum and Fusarium oxysporum. Molecules 26(9): 2462. doi: 10.3390/molecules26092462
Kumar A, Fitoussi N, Sanadhya P, et al. (2023) Two Candidate Meloidogyne javanica Effector Genes, MjShKT and MjPUT3: A Functional Investigation of Their Roles in Regulating Nematode Parasitism. Mol Plant Microbe Interact. 36(2): 79-94. doi: 10.1094/MPMI-10-22-0212-R
Lopes-Caitar VS, Nomura RBG, Hishinuma-Silva SM, et al. (2022) Time Course RNA-seq Reveals Soybean Responses against Root-Lesion Nematode and Resistance Players. Plants 11(21): 2983. doi: 10.3390/plants11212983
Lüdke D, Sakai T, Kourelis J, et al. (2023) A root-specific NLR network confers resistance to plant parasitic nematodes. bioRxiv 2023.12.14.571630; doi: 10.1101/2023.12.14.571630
Mahecha-Garnica S, Ye W, Schumacher LA, Gorny AM (2022) Soybean Cyst Nematode of Soybean: A Diagnostic Guide. Plant Health Progress 23: 507-513. doi: 10.1094/PHP-11-21-0138-DG
Mandal HR, Katel S, Subedi S, Shrestha J (2021) Plant Parasitic Nematodes and their management in crop production: a review. Journal of Agriculture and Natural Resources 4(2): 327–338. doi: 10.3126/janr.v4i2.33950
Margets A, Foster J, Kumar A, et al. (2024) The Soybean Cyst Nematode Effector Cysteine Protease 1 (CPR1) Targets a Mitochondrial Soybean Branched-Chain Amino Acid Aminotransferase (GmBCAT1). Mol Plant Microbe Interact. 37(11): 751-764. doi: 10.1094/MPMI-06-24-0068-R
Mattos VS, Leite RR, Santos MFA, et al. (2021) Genetic diversity of Meloidogyne spp. from rice and identification of multi-resistant sources in Oryza spp. accessions. Plant Pathol. 00: 1– 12. doi: 10.1111/ppa.13438
Mbatyoti A, De Beer A, Daneel MS, et al. (2021) The host status of glyphosate-tolerant soybean genotypes to Meloidogyne incognita and Pratylenchus infection. Trop. plant pathol. 46: 336–349. doi: 10.1007/s40858-020-00416-y
Mitchum MG, Hussey RS, Baum TJ, Wang X, Elling AA, Wubben M, Davis EL (2013) Nematode effector proteins: an emerging paradigm of parasitism. New Phytol 199: 879-894. doi: 10.1111/nph.12323
Mitkowski NA, Abawi GS (2003) Root-knot nematodes. The Plant Health Instructor. doi: 10.1094/PHI-I-2003-0917-01
, , , (2021) In-plant activation of root-specific expression of a cytotoxic gene disrupts the development of the root-knot nematode, Meloidogyne javanica. Plant Pathology 00: 1– 13. doi: 10.1111/ppa.13497
Piya S, Hawk T, Patel B, et al. (2021) Kinase-dead mutation: A novel strategy for improving soybean resistance to soybean cyst nematode Heterodera glycines. Molecular Plant Pathology 00: 1– 14. doi: 10.1111/mpp.13168
Sahebani N, Hadavi N (2008) Biological control of the root-knot nematode Meloidogyne javanica by Trichoderma harzianum. Soil Biology and Biochemistry 40: 2016-2020. doi: 10.1016/j.soilbio.2008.03.011
Sato K, Uehara T, Holbein J, et al. (2021) Transcriptomic Analysis of Resistant and Susceptible Responses in a New Model Root-Knot Nematode Infection System Using Solanum torvum and Meloidogyne arenaria. Front. Plant Sci. 12: 680151. doi: 10.3389/fpls.2021.680151
Sultana MS, Niyikiza D, Hawk TE, et al. (2024) Differential transcriptome reprogramming induced by the soybean cyst nematode Type 0 and Type 1.2.5.7 during resistant and susceptible interactions. Mol Plant Microbe Interact. doi: 10.1094/MPMI-08-24-0092-R
Sun M, Chen S, Kurle JE (2022) Interactive Effects of Soybean Cyst Nematode, Arbuscular-Mycorrhizal Fungi, and Soil pH on Chlorophyll Content and Plant Growth of Soybean. Phytobiomes Journal 6: 95-105. doi: 10.1094/PBIOMES-03-21-0024-R
Toju H, Tanaka Y (2019) Consortia of anti-nematode fungi and bacteria in the rhizosphere of soybean plants attacked by root-knot nematodes. R Soc Open Sci. 6(3): 181693. doi: 10.1098/rsos.181693
Tylka GL, Marett CC (2025) Known Distribution of the Soybean Cyst Nematode, Heterodera glycines, in the United States and Canada Through 2023. Plant Health Progress 26: 51-53. doi: 10.1094/PHP-08-24-0080-RS
, , , et al. (2023) Characterization and pathogenicity of Pratylenchus vandenbergae stat. nov. (Tylenchina: Pratylenchidae), a highly pathogenic root-lesion nematode parasitizing crops in Kenya and South Africa. Plant Pathology 00: 1–18. doi: 10.1111/ppa.13836
Wen TY, Wu XQ, Hu LJ, et al. (2021) A novel pine wood nematode effector, BxSCD1, suppresses plant immunity and interacts with an ethylene-forming enzyme in pine. Mol Plant Pathol. doi: 10.1111/mpp.13121
Wolfgang A, Taffner J, Guimarães RA, et al. (2019) Novel Strategies for Soil-Borne Diseases: Exploiting the Microbiome and Volatile-Based Mechanisms Toward Controlling Meloidogyne-Based Disease Complexes. Front. Microbiol. 10: 1296. doi: 10.3389/fmicb.2019.01296
Yang T, Xin Y, Liu T, et al. (2021) Bacterial volatile-mediated suppression of root-knot nematode (Meloidogyne incognita). Plant Disease. doi: 10.1094/PDIS-06-21-1139-RE
Yu S-F, Wang C-L, Hu Y-F, et al. (2022) Biocontrol of Three Severe Diseases in Soybean. Agriculture 12(9): 1391. doi: 10.3390/agriculture12091391
, , (2023) Metabolic variations in root tissues and rhizosphere soils of weak host plants potently lead to distinct host status and chemotaxis regulation of Meloidogyne incognita in intercropping. Molecular Plant Pathology 00: 1–16. doi: 10.1111/mpp.13396