.
Condición fitosanitaria: Presente
Grupo de cultivos: Cereales
Especie hospedante: Trigo (Triticum aestivum)
Rango de hospedantes: específico / estrecho (solo infecta trigo)
Epidemiología: policíclica, subaguda.
Etiología: Hongo. Necrotrófico (Hemibiotrófico)
Agente causal:
Zymoseptoria tritici (Desm.) Quaedvlieg & Crous 2011, Synonym: Septoria tritici Rob ex Desm (anamorfo),
Mycosphaerella graminicola (Fuckel) J. Schröt., (1894) (teleomorfo)
.
Taxonomía: Eukaryota > Fungi > Dikarya > Ascomycota > Pezizomycotina > Dothideomycetes > Capnodiales > Mycosphaerellaceae > Zymoseptoria
.
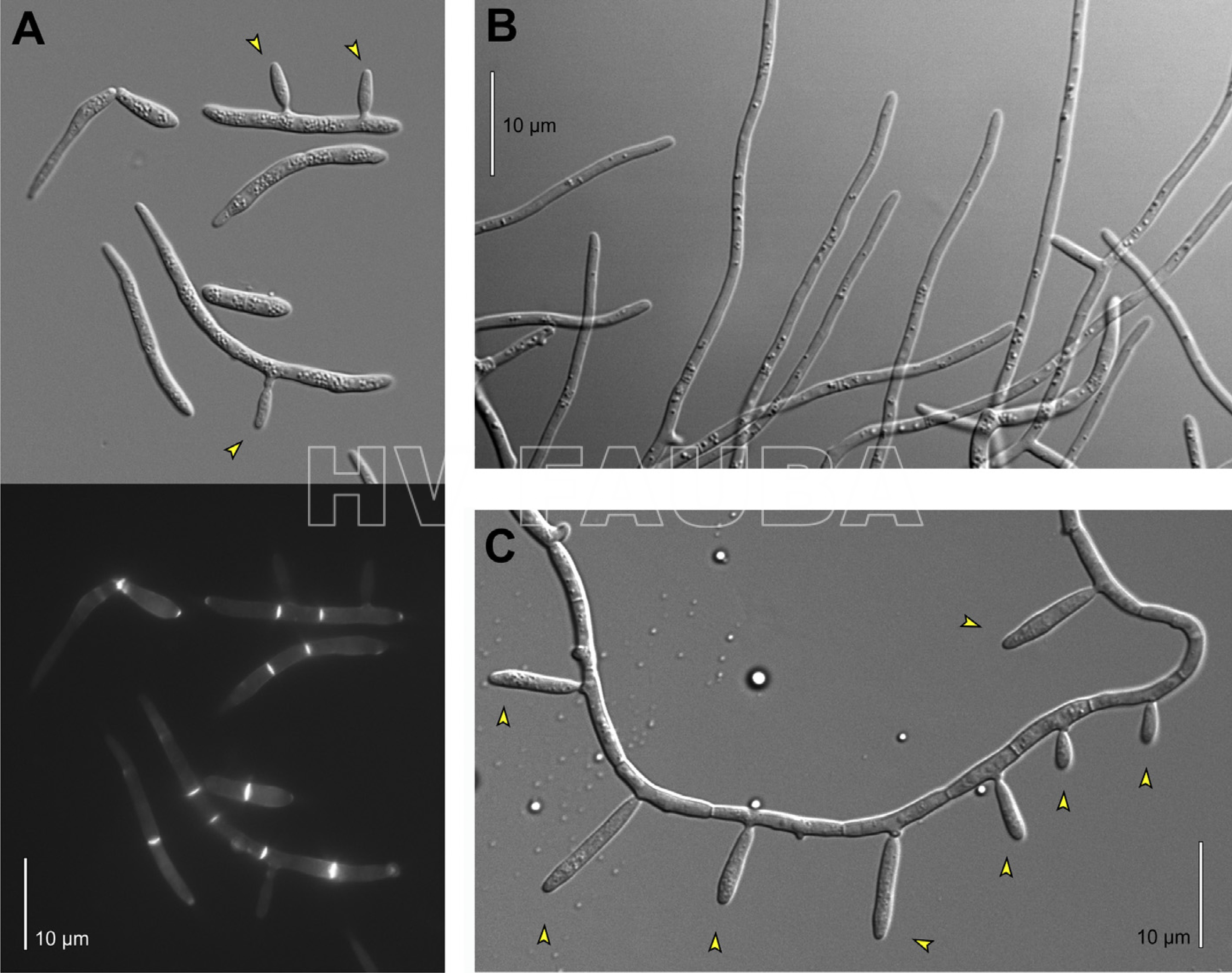
- Formas de crecimiento de Z. tritici. (A) Esporas a partir de picnidios, multicelulares y forman cogollos laterales (punta de flecha abierta) y terminales (punta de flecha llena). La barra de escala representa 10 um. (B) Hifas de Z. tritici. La barra de escala representa 10 um. (C) Micropycnidia que están surgiendo de las células hifales. La barra de escala representa 10 um. Autor: Steinberg, 2015.
.
.
.
Síntomas y signos
Las manchas surgen primeramente como puntos cloróticos que luego se expanden, formando las típicas manchas necróticas elongadas, con apariencia de “parches pajizos”. Sobre las mismas aparecen “salpicados” números puntos oscuros (picnidios). Puede provocar la muerte de las hojas. Afecta principalmente las hojas y vainas. Rara vez puede atacar las espigas. En general, las hojas basales presentan mayor intensidad de síntomas. Se evidencia un crecimiento vertical.
.
- Autor: Maccheek
- Síntomas y signo (puntuaciones negras) de la Septoriosis de la hoja de trigo. Autor: Corteva Agriscience
- Picnidio subepidérmico de Zymoseptoria tritici. Autor: Corteva Agriscience
- Cirro de Zymoseptoria tritici. Autor: Corteva Agriscience
- Cirro de Zymoseptoria tritici. Autor: Corteva Agriscience
- Conidio germinado de Zymoseptoria tritici y tubo germinativo penetrando por estoma. Autor: Corteva Agriscience
.
Ciclo de la enfermedad y epidemiología
La principal fuente de inóculo es el rastrojo infectado (principalmente con picnidios). Para la liberación de los conidios, los picnidios requieren la presencia de agua libre. El transporte de las esporas es siempre por salpicaduras de gotas de lluvia las que son llevadas por el viento a corta distancia. La temperatura óptima para la infección es de 15‑20ºC, y con 72‑96 horas de mojado. El patrón de distribución en el lote es generalizada y uniforme. Existe poca información sobre su presencia y transmisión en semillas. En Argentina la importancia de hospedantes secundarios, como fuentes de inóculo, no ha sido esclarecida. En caso de producirse la reproducción sexual, las ascosporas se forman dentro de pseudotecios.
Zymoseptoria tritici infecta la planta hospedante casi exclusivamente a través de los estomas de las hojas. Z. tritici se diferencia de otros hongos fitopatógenos en que no produce estructuras activas especializadas durante la infección y se limita al espacio apoplástico de la planta hospedante.
.
- Síntomas y signo, puntuaciones negras (picnidios de Zymoseptoria tritici). Autor: Frédéric Suffert
.
Condiciones predisponentes
para el establecimiento y dispersión de la enfermedad se requieren períodos prolongados de elevada humedad relativa, neblinas, frecuentes lluvias, lloviznas y temperaturas entre 15 y 20 °C durante período de cultivo. El monocultivo asegura la presencia indefinida del patógeno en el cultivo. Resulta más grave bajo siembra directa.
.
Daños
Las pérdidas más comunes son del 20 %, pero se citan reducciones del rendimiento del orden del 40 al 60% según los cultivares. Entre los componentes más afectados se encuentran el peso y el número de granos por metro cuadrado.
.
Manejo Integrado
La principal medida preferencial es la siembra de variedades resistentes, si hubiera disponibles; y en segundo lugar la rotación de cultivos.
* Rotación de cultivos: El trigo debería ser cultivado en el mismo lote, sólo después de la completa mineralización de los restos culturales. Rotaciones con colza, lino y avena resultan exitosas para el manejo de la enfermedad.
Otras:
* Aplicación de fungicidas foliares: Esta práctica es recomendada en cultivos donde la enfermedad ya está presente alcanzando un nivel de daño que justifique el control químico. Entre los fungicidas recomendados los más eficientes son los triazoles sistémicos.
* Eliminación de plantas guachas: Tales plantas garantizan la supervivencia del patógeno en el verano‑otoño, actuando como puentes «verdes» y comprometiendo el período de rotación necesario.
* Resistencia varietal: La mayoría de los cultivares de trigo son susceptibles en diversos grados a Septoria, aunque existen algunos genotipos de trigo que tienen mejor comportamiento.
* Siembra de mezclas de cultivares con diferente nivel de resistencia: en Túnez la inclusión de un cultivar resistente a enfermedades en una proporción del 25% con un cultivar susceptible proporcionó una reducción sustancial de casi el 50% en la gravedad de la enfermedad en comparación con el rodal puro susceptible (Ben et al., 2020).
.
.
Videos
Timing of control for Septoria tritici blotch
Importance of septoria control
.
Links
Septoria tritici blotch network
Unique resistance gene to leaf spot disease for the first time successfully introduced in wheat, January 19, 2021
.
.
.
.
Bibliografía
Ajaz S, Benbow HR, Christodoulou T, et al. (2021) Evaluation of the susceptibility of modern, wild, ancestral, and mutational wheat lines to Septoria tritici blotch disease. Plant Pathol. doi: 10.1111/ppa.13369
Ajigboye OO, Jayaweera DP, Angelopoulou D, et al. (2021) The role of photoprotection in defence of two wheat genotypes against Zymoseptoria tritici. Plant Pathology. Accepted Author Manuscript. doi: 10.1111/ppa.13392
Amezrou R, Audéon C, Compain J, et al. (2022) A secreted protease-like protein in Zymoseptoria tritici is responsible for avirulence on Stb9 resistance gene in wheat. bioRxiv 2022.10.31.514577; doi: 10.1101/2022.10.31.514577
Andersson B, Djurle A, Ørum JE, et al. (2022) Comparison of models for leaf blotch disease management in wheat based on historical yield and weather data in the Nordic-Baltic region. Agron. Sustain. Dev. 42: 42. doi: 10.1007/s13593-022-00767-7
Ballu A, Ugazio C, Duplaix C, et al. (2022) Preventing multiple resistance above all: new insights for managing fungal adaptation. bioRxiv 2022.12.17.520869. doi: 10.1101/2022.12.17.520869
Barroso-Bergadà D, Massot M, Vignolles N, et al. (2023) Metagenomic Next-Generation Sequencing (mNGS) Data Reveal the Phyllosphere Microbiome of Wheat Plants Infected by the Fungal Pathogen Zymoseptoria tritici. Phytobiomes Journal 7: 281-287. doi: 10.1094/PBIOMES-02-22-0008-FI
Battistini G, Gazzetti K, Collina M (2022) A New Approach: Determining cyt b G143A Allele Frequency in Zymoseptoria tritici by Digital Droplet PCR. Biology 11(2): 240. doi: 10.3390/biology11020240
Bellah H, Gazeau G, Gélisse S, et al. (2023) A highly multiplexed assay to monitor pathogenicity, fungicide resistance and gene flow in the fungal wheat pathogen Zymoseptoria tritici. PLoS ONE 18(2): e0281181. doi: 10.1371/journal.pone.0281181
, , , et al. (2020) Improved control of septoria tritici blotch in durum wheat using cultivar mixtures. Plant Pathology 69: 1655– 1665. doi: 10.1111/ppa.13247
, , , (2023) Specific virulence patterns in Tunisian Zymoseptoria tritici strains isolated from bread and durum wheat. Plant Pathology 00: 1– 13. doi: 10.1111/ppa.13710
Birr T, Hasler M, Verreet J-A, Klink H (2021) Temporal Changes in Sensitivity of Zymoseptoria tritici Field Populations to Different Fungicidal Modes of Action. Agriculture 11(3): 269. doi: 10.3390/agriculture11030269
Bocquet L, Rivière C, Dermont C, et al. (2018) Antifungal activity of hop extracts and compounds against the wheat pathogen Zymoseptoria tritici. Industrial Crops and Products 122: 290-297. doi: 10.1016/j.indcrop.2018.05.061
Brennan CJ, Zhou B, Benbow HR, et al. (2020) Taxonomically Restricted Wheat Genes Interact With Small Secreted Fungal Proteins and Enhance Resistance to Septoria Tritici Blotch Disease. Front. Plant Sci. 11: 433. doi: 10.3389/fpls.2020.00433
Brown JKM, Chartrain L, Lasserre-Zuber P, et al. (2015) Genetics of resistance to Zymoseptoria tritici and applications to wheat breeding. Fungal Genetics and Biology 79: 33-41. doi: 10.1016/j.fgb.2015.04.017
Brunner PC, Stefanato FL, McDonald BA (2008) Evolution of the CYP51 gene in Mycosphaerella graminicola: evidence for intragenic recombination and selective replacement. Mol Plant Pathol. 9: 305–316. doi: 10.1111/j.1364-3703.2007.00464.x
Brunner PC, McDonald BA (2018) Evolutionary analyses of the avirulence effector AvrStb6 in global populations of Zymoseptoria tritici identify candidate amino acids involved in recognition. Molecular Plant Pathology. doi: 10.1111/mpp.12662
Campanaro A, Srivastava AK, Zhang C, et al. (2021), TaWRKY10 transcription factor is a novel jasmonic acid signalling regulator involved in immunity against Septoria tritici blotch disease in wheat. Plant Pathol. doi: 10.1111/ppa.13388
Castro AC, Simón MR (2016) Effect of tolerance to Septoria tritici blotch on grain yield, yield components and grain quality in Argentinean wheat cultivars. Crop Protection 90: 66-76. doi: 10.1016/j.cropro.2016.08.015
Chaudhari Y, Cairns TC, Sidhu Y, et al. (2019) The Zymoseptoria tritici ORFeome: A Functional Genomics Community Resource. Mol Plant Microbe Interact. 32(12): 1564-1570. doi: 10.1094/MPMI-05-19-0123-A
Chen H, King R, Smith D, et al. (2023) Combined pangenomics and transcriptomics reveals core and redundant virulence processes in a rapidly evolving fungal plant pathogen. BMC Biol 21: 24. doi: 10.1186/s12915-023-01520-6
Cheval P, Siah A, Bomble M, et al. (2017) Evolution of QoI resistance of the wheat pathogen Zymoseptoria tritici in Northern France. Crop Protection 92: 131-133. doi: 10.1016/j.cropro.2016.10.017
, , (2023) Comparison of the impact of two key fungal signalling pathways on Zymoseptoria tritici infection reveals divergent contribution to invasive growth through distinct regulation of infection-associated genes. Molecular Plant Pathology 24: 1220–1237. doi: 10.1111/mpp.13365
Cools HJ, Mullins JG, Fraaije BA, et al. (2011) Impact of recently emerged sterol 14a-demethylase (CYP51) variants of Mycosphaerella graminicola on azole fungicide sensitivity. Appl. Environ. Microbiol. 77: 3830–3837. doi: 10.1128/AEM.00027-11
Cools HJ, Bayon C, Atkins S, et al. (2012) Overexpression of the sterol 14α-demethylase gene (MgCYP51) in Mycosphaerella graminicola isolates confers a novel azole fungicide sensitivity phenotype. Pest Manag. Sci. 68: 1034-1040. doi: 10.1002/ps.3263
Cools HJ, Fraaije BA (2013) Update on mechanisms of azole resistance in Mycosphaerella graminicola and implications for future control. Pest Manag Sci. 69: 150-155. doi: 10.1002/ps.3348
Crawford LA, Cuzzucoli Crucitti V, Stimpson A, et al. (2023) A potential alternative to fungicides using actives-free (meth)acrylate polymers for protection of wheat crops from fungal attachment and infection. Green Chem 25(21): 8558-8569. doi: 10.1039/d3gc01911j
Curvers K, Pycke B, Kyndt T, et al. (2014) Sensitivity towards DMI fungicides and haplotypic diversity of their CYP51 target in the Mycosphaerella graminicola population of Flanders. J. Plant Dis. Prot. 121: 156–163. doi: 10.1007/BF03356504
Dalvand M, Soleimani Pari MJ, Zafari D (2017) Evaluating the efficacy of STB resistance genes to Iranian Zymoseptoria tritici isolates. Journal of Plant Diseases and Protection: 1–6. doi: 10.1007/s41348-017-0143-3
Dooley H, Shaw MW, Mehenni‐Ciz J, Spink J, Kildea S (2016) Detection of Zymoseptoria tritici SDHI‐insensitive field isolates carrying the SdhC‐H152R and SdhD‐R47W substitutions. Pest. Manag. Sci. 72: 2203-2207. doi: 10.1002/ps.4269
, , (2023) Bacterial predation of a fungal wheat pathogen: Prelude to experimental evolution of enhanced biocontrol agents. Plant Pathology 00: 1– 10. doi: 10.1111/ppa.13716
Estep LK, Zala M, Anderson NP, et al. (2013) First Report of Resistance to QoI Fungicides in North American Populations of Zymoseptoria tritici, Causal Agent of Septoria Tritici Blotch of Wheat. Plant Disease 97(11): 1511. doi: 10.1094/PDIS-05-13-0486-PDN
Estep LK, Torriani SFF, Zala M, et al. (2015) Emergence and early evolution of fungicide resistance in North American populations of Zymoseptoria tritici. Plant Pathology 64: 961-971. doi: 10.1111/ppa.12314
(1999) The Septoria tritici and Stagonospora nodorum blotch diseases of wheat. European Journal of Plant Pathology 105: 629–41. doi: 10.1023/A:1008716812259
Fedoryshchak RO, Ocasio CA, Strutton B, et al. (2020) Wheat pathogen Zymoseptoria tritici N-myristoyltransferase inhibitors: on-target antifungal activity and an unusual metabolic defense mechanism. RSC Chem Biol. 1(2): 68-78. doi: 10.1039/D0CB00020E
Ferjaoui S, Aouini L, Slimane RB, et al. (2022) Deciphering resistance to Zymoseptoria tritici in the Tunisian durum wheat landrace accession ‘Agili39’. BMC Genomics 23: 372. doi: 10.1186/s12864-022-08560-2
Feurtey A, Lorrain C, McDonald MC, et al. (2022) A thousand-genome panel retraces the global spread and climatic adaptation of a major crop pathogen. bioRxiv 2022.08.26.505378; doi: 10.1101/2022.08.26.505378
Ficke A, Cowger C, Bergstrom G, Brodal G (2018) Understanding Yield Loss and Pathogen Biology to Improve Disease Management: Septoria Nodorum Blotch – A Case Study in Wheat. Plant Disease 102(4): 696-707. doi: 10.1094/PDIS-09-17-1375-FE
Fones H, Gurr S (2015) The impact of Septoria tritici Blotch disease on wheat: An EU perspective. Fungal Genetics and Biology 79: 3-7. doi: 10.1016/j.fgb.2015.04.004
Fouché G, Michel T, Lalève A, et al. (2022) Directed evolution predicts cytochrome b G37V target site modification as probable adaptive mechanism towards the QiI fungicide fenpicoxamid in Zymoseptoria tritici. Environ Microbiol. 24: 1117-1132. doi: 10.1111/1462-2920.15760
Fraaije BA, Cools HJ, Kim S-H, et al. (2007) A novel substitution I381V in the sterol 14α-demethylase (CYP51) of Mycosphaerella graminicola is differentially selected by azole fungicides. Mol. Plant Pathol. 8: 245–254. doi: 10.1111/j.1364-3703.2007.00388.x
Fraaije BA, Bayon C, Atkins S, et al. (2012) Risk assessment studies on succinate dehydrogenase inhibitors, the new weapons in the battle to control Septoria leaf blotch in wheat. Molecular Plant Pathology, 13: 263-275. doi: 10.1111/j.1364-3703.2011.00746.x
Francisco CS, Ma X, Zwyssig MM, et al. (2019) Morphological changes in response to environmental stresses in the fungal plant pathogen Zymoseptoria tritici. Scientific Reports 9: 9642. doi: 10.1038/s41598-019-45994-3
Garnault M, Duplaix C, Leroux P, et al. (2021) Large‐scale study validates that regional fungicide applications are major determinants of resistance evolution in the wheat pathogen Zymoseptoria tritici in France. New Phytol. doi: 10.1111/nph.17107
Grandaubert J, Dutheil JY, Stukenbrock EH (2017) The genomic rate of adaptation in the fungal wheat pathogen Zymoseptoria tritici. bioRxiv 176727; doi: doi: 10.1101/176727
Grasso V, Palermo S, Sierotzki H, et al. (2006) Cytochrome b gene structure and consequences for resistance to Qo inhibitor fungicides in plant pathogens. Pest Manag. Sci. 62(6): 465-472. doi: 10.1002/ps.1236
Gutiérrez-Alonso O, Hawkins NJ, Cools HJ, et al. (2017) Dose-dependent selection drives lineage replacement during the experimental evolution of SDHI fungicide resistance in Zymoseptoria tritici. Evol Appl. 10(10): 1055-1066. doi: 10.1111/eva.12511
Gutierrez Vazquez Y, Adams IP, McGreig S, et al. (2022) Profiling azole resistant haplotypes within Zymoseptoria tritici populations using nanopore sequencing. Front. Agron. 4: 943440. doi: 10.3389/fagro.2022.943440
Hagerty CH, Klein AM, Reardon CL, et al. (2021) Baseline and Temporal Changes in Sensitivity of Zymoseptoria tritici Isolates to Benzovindiflupyr in Oregon, U.S.A., and Cross-Sensitivity to Other SDHI Fungicides. Plant Disease 105(1): 169-174. doi: 10.1094/PDIS-10-19-2125-RE
Harrat W , Neddaf HM, Keddad A, Bouznad Z (2017) First report of the Zymoseptoria tritici teleomorph stage causing septoria leaf blotch of wheat in Algeria. New Disease Reports 35: 30. doi: 10.5197/j.2044-0588.2017.035.030
Hassine M, Siah A, Hellin P, et al. (2019) Sexual reproduction of Zymoseptoria tritici on durum wheat in Tunisia revealed by presence of airborne inoculum, fruiting bodies and high levels of genetic diversity. Fungal Biology 123: 763-772. doi: 10.1016/j.funbio.2019.06.006
Hassine M, Bnejdi F, Bahri BA, et al. (2022) Detection of Maternal and Cytoplasmic Effects on Resistance to Zymoseptoria tritici in Durum Wheat. BioMed Research International vol. 2022, Article ID 8497417. doi: 10.1155/2022/8497417
Heick TM, Justesen AF, Jørgensen LN (2017) Resistance of wheat pathogen Zymoseptoria tritici to DMI and QoI fungicides in the Nordic-Baltic region – a status. Eur. J. Plant Pathol. 149: 669–682. doi: 10.1007/s10658-017-1216-7
Heick TM, Justesen AF, Jørgensen LN (2017) Anti-resistance strategies for fungicides against wheat pathogen Zymoseptoria tritici with focus on DMI fungicides. Crop Protection 99: 108-117. doi: 10.1016/j.cropro.2017.05.009
Heick TM, Matzen N, Jørgensen LN (2020) Reduced field efficacy and sensitivity of demethylation inhibitors in the Danish and Swedish Zymoseptoria tritici populations. Eur J Plant Pathol 157: 625–636. doi: 10.1007/s10658-020-02029-2
, , , et al. (2020) Multiplex qPCR assay for simultaneous quantification of CYP51‐S524T and SdhC‐H152R substitutions in European populations of Zymoseptoria tritici. Plant Pathology 69: 1666– 1677. doi: 10.1111/ppa.13252
Hellin P, Duvivier M, Heick MT, et al. (2021) Spatio-temporal distribution of DMI and SDHI fungicide resistance of Zymoseptoria tritici throughout Europe based on frequencies of key target-site alterations. Pest Manag Sci. 7: 5576-5588. doi: 10.1002/ps.6601
Hoorne C, Lamari L, Ballance GM, Gilbert J (2002) First report of Mycosphaerella graminicola, the sexual state of Septoria tritici, in Manitoba, Canada. Canadian Journal of Plant Pathology 24: 445-449. doi: 10.1080/07060660209507032
Huf A, Rehfus A, Lorenz KH, et al. (2018) Proposal for a new nomenclature for CYP51 haplotypes in Zymoseptoria tritici and analysis of their distribution in Europe. Plant Pathology 67: 1706-1712. doi: 10.1111/ppa.12891
International Wheat Genome Sequencing Consortium (IWGSC); IWGSC RefSeq principal investigators:, Appels R, et al. (2018) Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science 361(6403): eaar7191. doi: 10.1126/science.aar7191
Jørgensen LN, Matzen N, Hansen JG, et al. (2018) Four azoles’ profile in the control of Septoria, yellow rust and brown rust in wheat across Europe. Crop Protection 503: 105, 16-27. doi: 10.1016/j.cropro.2017.10.018
Jørgensen LN, Matzen N, Heick TM, et al. (2021) Decreasing azole sensitivity of Z. tritici in Europe contributes to reduced and varying field efficacy. J. Plant Dis. Prot. 128: 287–301. doi: 10.1007/s41348-020-00372-4
Jørgensen LN, Matzen N, Heick TM, et al. (2022) Shifting sensitivity of septoria tritici blotch compromises field performance and yield of main fungicides in Europe. Front. Plant Sci. 13: 1060428. doi: 10.3389/fpls.2022.1060428
Karisto P, Hund A, Yu K, et al. (2018) Ranking Quantitative Resistance to Septoria tritici Blotch in Elite Wheat Cultivars Using Automated Image Analysis. Phytopathology 108(5): 568-581. doi: 10.1094/PHYTO-04-17-0163-R
Karisto P, Suffert F, Mikaberidze A (2022) Measuring Splash Dispersal of a Major Wheat Pathogen in the Field. PhytoFrontiers™ 2: 30-40. doi: 10.1094/PHYTOFR-05-21-0039-R
Keller B, Krattinger SG (2018) A new player in race-specific resistance. Nature Plants (online). doi: 10.1038/s41477-018-0126-9
Kellner R, Bhattacharyya A, Poppe S, et al. (2014) Expression profiling of the wheat pathogen Zymoseptoria tritici reveals genomic patterns of transcription and host-specific regulatory programs. Genome Biol Evol. 6(6): 1353-65. doi: 10.1093/gbe/evu101
Kema GHJ, Mirzadi Gohari A, et al. (2018) Stress and sexual reproduction affect the dynamics of the wheat pathogen effector AvrStb6 and strobilurin resistance. Nature Genetics: 1546-1718. doi: 10.1038/s41588-018-0052-9
Kettles GJ, Kanyuka K (2016) Dissecting the Molecular Interactions between Wheat and the Fungal Pathogen Zymoseptoria tritici. Front. Plant Sci. 7: 508. doi: 10.3389/fpls.2016.00508
Kettles GJ, Bayon C, Sparks CA, et al. (2018) Characterization of an antimicrobial and phytotoxic ribonuclease secreted by the fungal wheat pathogen Zymoseptoria tritici. New Phytol. 217(1): 320-331. doi: 10.1111/nph.14786
Kiiker R, Juurik M, Heick TM, Mäe A (2021) Changes in DMI, SDHI, and QoI Fungicide Sensitivity in the Estonian Zymoseptoria tritici Population between 2019 and 2020. Microorganisms 9(4): 814. doi: 10.3390/microorganisms9040814
Kilaru S, Schuster M, Latz M, Guo M, Steinberg G (2015) Fluorescent markers of the endocytic pathway in Zymoseptoria tritici. Fungal Genetics and Biology 79: 150-157. doi: 10.1016/j.fgb.2015.03.019
Kilarua S, Schuster M, Ma W, Steinberg G (2017) Fluorescent markers of various organelles in the wheat pathogen Zymoseptoria tritici. Fungal Genetics and Biology 105: 16-27. doi: 10.1016/j.fgb.2017.05.001
Kildea S, Dunne B, Mullins E (2010) Pyraclostrobin reduces germ tube growth of QoI-resistant Mycosphaerella graminicola pycnidiospores and the severity of septoria tritici blotch on winter wheat. Plant Pathology 59: 1091-1098. doi: 10.1111/j.1365-3059.2010.02348.x
Kildea S, Mehenni-Ciz J, Spink J, O’Sullivan E (2014) Changes in the frequency of Irish Mycophaerella graminicola CYP51 variants 2006–2011. In Dehne HW, Deising HB, Fraaije B, et al. (Eds.), 17th international Reinhardsbrunn symposium (pp. 143–144). DPG Spectrum Phytomedizin: Braunschweig, Germany.
Kildea S, Marten-Heick T, Grant J, et al. (2019) A combination of target-site alterations, overexpression and enhanced efflux activity contribute to reduced azole sensitivity present in the Irish Zymoseptoria tritici population. Eur. J. Plant Pathol. 154: 529-540. doi: 10.1007/s10658-019-01676-4
Kildea S, Bucur D, Hutton F, et al. (2019) Prevalence of QoI resistance and mtDNA diversity in the Irish Zymoseptoria tritici population. Irish J. Agric. Food Res. 58: 27-33. doi: 10.2478/ijafr-2019-0004
Kildea S, Sheppard L, Cucak M, Hutton F (2021) Detection of virulence to septoria tritici blotch (STB) resistance conferred by the winter wheat cultivar Cougar in the Irish Zymoseptoria tritici population and potential implications for STB control. Plant Pathol. 70: 2137– 2147. doi: 10.1111/ppa.13432
Kildea S, Hellin P, Heick TM, Hutton F (2022) Baseline sensitivity of European Zymoseptoria tritici populations to the complex III respiration inhibitor fenpicoxamid. Pest Manag Sci. doi: 10.1002/ps.7067
Kildea S, Hellin P, Heick TM, Hutton F (2022) Baseline sensitivity of European Zymoseptoria tritici populations to the complex III respiration inhibitor fenpicoxamid. Pest Manag Sci. 78(11): 4488-4496. doi: 10.1002/ps.7067
Klink H, Verreet J-A, Hasler M, Birr T (2021) Will Triazoles Still Be of Importance in Disease Control of Zymoseptoria tritici in the Future? Agronomy 11(5): 933. doi: 10.3390/agronomy11050933
Klink H, Prahl KC, Hasler M, et al. (2022) Efficiency and Effectivity of a Biological–Epidemiological Fungal Disease Management System in Wheat—A Study of 26 Years. Agriculture 12(8): 1099. doi: 10.3390/agriculture12081099
Kristoffersen R, Eriksen LB, Nielsen GC, et al. (2022) Management of Septoria Tritici Blotch Using Cultivar Mixtures. Plant Disease 106(5): 1341-1349. doi: 10.1094/PDIS-01-21-0069-RE
Lendenmann MH, Croll D, Stewart EL, McDonald BA (2014) Quantitative trait locus mapping of melanization in the plant pathogenic fungus Zymoseptoria tritici. G3 (Bethesda) 4(12): 2519-33. doi: 10.1534/g3.114.015289
Leroux P, Albertini C, Gautier A, et al. (2007) Mutations in the CYP51 gene correlated with changes in sensitivity to sterol 14 alpha-demethylation inhibitors in field isolates of Mycosphaerelia graminicola. Pest Management Science 63(7): 688–698. doi: 10.1002/ps.1390
Leroux P, Walker A-S (2011) Multiple mechanisms account for resistance to sterol 14α-demethylation inhibitors in field isolates of Mycosphaerella graminicola. Pest. Manag. Sci. 67: 44-59. doi: 10.1002/ps.2028
Louriki S, Rehman S, El Hanafi S, et al. (2021) Identification of Resistance Sources and Genome-Wide Association Mapping of Septoria Tritici Blotch Resistance in Spring Bread Wheat Germplasm of ICARDA. Front. Plant Sci. 12: 600176. doi: 10.3389/fpls.2021.600176
Ma X, Keller B, McDonald BA, et al. (2017) Comparative transcriptomics reveals how wheat responds to infection by Zymoseptoria tritici. Molecular Plant-Microbe Interactions (accepted). doi: 10.1094/MPMI-10-17-0245-R
Mäe A, Fillinger S, Sooväli P and Heick TM (2020) Fungicide Sensitivity Shifting of Zymoseptoria tritici in the Finnish-Baltic Region and a Novel Insertion in the MFS1 Promoter. Front. Plant Sci. 11: 385. doi: 10.3389/fpls.2020.00385
McCorison CB, Goodwin SB (2020) The wheat pathogen Zymoseptoria tritici senses and responds to different wavelengths of light. BMC Genomics 21: 513. doi: 10.1186/s12864-020-06899-y
McDonald MC, McDonald BA and Solomon PS (2015) Recent advances in the Zymoseptoria tritici–wheat interaction: insights from pathogenomics. Frontiers in Plant Science 6: 102. doi: 10.3389/fpls.2015.00102
McDonald BA, Mundt CC (2016) How knowledge of pathogen population biology informs management of Septoria tritici blotch. Phytopathology 106: 948–955. doi: 10.1094/PHYTO-03-16-0131-RVW
Mehrabi R, Makhdoomi A, Jafar-Aghaie M (2015) Identification of New Sources of Resistance to Septoria Tritici Blotch Caused by Zymoseptoria tritici. J Phytopathol 163: 84–90. doi: 10.1111/jph.12282
Meile L, Croll D, Brunner PC, et al. (2018) A fungal avirulence factor encoded in a highly plastic genomic region triggers partial resistance to septoria tritici blotch. New Phytologist. doi: 10.1111/nph.15180
Morais D, Sache I, Suffert F, Laval V (2016) Is the onset of septoria tritici blotch epidemics related to the local pool of ascospores?. Plant Pathol 65: 250-260. doi: 10.1111/ppa.12408
Morais D, Duplaix C, Sache I, et al. (2019) Overall stability in the genetic structure of a Zymoseptoria tritici population from epidemic to interepidemic stages at a small spatial scale. Eur J Plant Pathol 154: 423–436. doi: 10.1007/s10658-018-01666-y
Oggenfuss U, Badet T, Wicker T, et al. (2021) A population-level invasion by transposable elements triggers genome expansion in a fungal pathogen. Elife. 10: e69249. doi: 10.7554/eLife.69249
Omrane S, Sghyer H, Audéon C, et al. (2015) Fungicide efflux and the MgMFS1 transporter contribute to the multidrug resistance phenotype in Zymoseptoria tritici field isolates. Environ Microbiol.17(8): 2805-2823. doi: 10.1111/1462-2920.12781
Omrane S, Audeon C, Ignace A, et al. (2017) Plasticity of the MFS1 promoter leads to multi drug resistance in the wheat pathogen Zymoseptoria tritici. bioRxiv 174722; doi: 10.1101/174722
, , , (2021) Annual dynamics of Zymoseptoria tritici populations in wheat cultivar mixtures: A compromise between the efficacy and durability of a recently broken-down resistance gene? Plant Pathology 00: 1– 15. doi: 10.1111/ppa.13458
, , , et al. (2022) Multiple scenarios for sexual crosses in the fungal pathogen Zymoseptoria tritici on wheat residues: potential consequences for virulence gene transmission. bioRxiv 2022.02.24.481803; doi: 10.1101/2022.02.24.481803
Owen WJ, Yao C, Myung K, et al. (2017) Biological characterization of fenpicoxamid, a new fungicide with utility in cereals and other crops. Pest. Manag. Sci, 73: 2005-2016. doi: 10.1002/ps.4588
Phan HTT, Rybak K, Bertazzoni S, et al. (2018) Novel sources of resistance to Septoria nodorum blotch in the Vavilov wheat collection identified by genome-wide association studies. Theoretical and Applied Genetics 1–16. doi: 10.1007/s00122-018-3073-y
Plissonneau C, Hartmann FE, Croll D (2018) Pangenome analyses of the wheat pathogen Zymoseptoria tritici reveal the structural basis of a highly plastic eukaryotic genome. BMC Biology 16(1): 5. doi: 10.1186/s12915-017-0457-4
Poppe S (2014) Host specialization in the fungal plant pathogen Zymoseptoria tritici. Doctoral thesis, Philipps-University Marburg, Germany, Link
Prahl KC, Klink H, Hasler M, et al. (2022) Can Decision Support Systems Help Improve the Sustainable Use of Fungicides in Wheat? Sustainability 14(23): 15599. doi: 10.3390/su142315599
Quaedvlieg W, Kema GH, Groenewald JZ, et al. (2011) Zymoseptoria gen. nov.: a new genus to accommodate Septoria-like species occurring on graminicolous hosts. Persoonia 26: 57-69. doi: 10.3767/003158511X571841
Rahman A, Doohan F, Mullins E (2020) Quantification of In Planta Zymoseptoria tritici Progression Through Different Infection Phases and Related Association with Components of Aggressiveness. Phytopathology 110(6): 1208-1215. doi: 10.1094/PHYTO-09-19-0339-R
Rehfus A, Strobel D, Bryson R, Stammler G (2018) Mutations in sdh genes in field isolates of Zymoseptoria tritici and impact on the sensitivity to various succinate dehydrogenase inhibitors. Plant Pathology 67: 175–180. doi: 10.1111/ppa.12715
Ronis A, Jørgensen LN, Semaškienė R, et al. (2014) Sensitivity of Mycosphaerella graminicola isolates to demethylation-inhibiting (DMI) fungicides. Zemdirbyste-Agriculture 101 (2): 177–184. doi: 10.13080/z-a.2014.101.023
Rossato Augusti G (2019) The status of fungicide resistance in South American Zymoseptoria tritici populations. PhD Thesis, University of Reading. Link
Saintenac C, Lee WS, Cambon F, et al. (2018) Wheat receptor-kinase-like protein Stb6 controls gene-for-gene resistance to fungal pathogen Zymoseptoria tritici. Nature Genetics 50(3): 368-374. doi: 10.1038/s41588-018-0051-x
Saintenac C, Cambon F, Aouini L, et al. (2021) A wheat cysteine-rich receptor-like kinase confers broad-spectrum resistance against Septoria tritici blotch. Nature Communications 12: 433. doi: 10.1038/s41467-020-20685-0
Samain E, Aussenac T, Selim S (2019) The Effect of Plant Genotype, Growth Stage, and Mycosphaerella graminicola Strains on the Efficiency and Durability of Wheat-Induced Resistance by Paenibacillus sp. Strain B2. Front. Plant Sci. 10: 587. doi: 10.3389/fpls.2019.00587
Samils B, Andersson B, Edin E, et al. (2021) Development of a PacBio Long-Read Sequencing Assay for High Throughput Detection of Fungicide Resistance in Zymoseptoria tritici. Front. Microbiol. 12: 692845. doi: 10.3389/fmicb.2021.692845
Scalliet G, Bowler J, Luksch T, et al. (2012) Mutagenesis and Functional Studies with Succinate Dehydrogenase Inhibitors in the Wheat Pathogen Mycosphaerella graminicola. PLoS ONE 7(4): e35429. doi: 10.1371/journal.pone.0035429
Seybold H, Demetrowitsch TJ, Hassani MA, et al. (2020) A fungal pathogen induces systemic susceptibility and systemic shifts in wheat metabolome and microbiome composition. Nat Commun 11, 1910. doi: 10.1038/s41467-020-15633-x
Siah A, BombleM, Tisserant B, et al. (2018) Genetic structure of Zymoseptoria tritici in northern France at region, field, plant and leaf layer scales. Phytopathology (accepted). doi: 10.1094/PHYTO-09-17-0322-R
Singh NK, Badet T, Abraham L, et al. (2021) Rapid sequence evolution driven by transposable elements at a virulence locus in a fungal wheat pathogen. BMC Genomics 22: 393. doi: 10.1186/s12864-021-07691-2
Skinner W, Bailey A, Renwick A, et al. (1998) A single amino-acid substitution in the iron-sulphur protein subunit of succinate dehydrogenase determines resistance to carboxin in Mycosphaerella graminicola. Curr Genet. 34(5): 393-8. doi: 10.1007/s002940050412
, , , (2022) Higher alterations in leaf fluorescence parameters of wheat cultivars predict more extensive necrosis in response to Zymoseptoria tritici. Plant Pathology 71: 1454– 1466. doi: 10.1111/ppa.13569
Steinberg G (2015) Cell biology of Zymoseptoria tritici: Pathogen cell organization and wheat infection. Fungal Genetics and Biology 79: 17-23. doi: 10.1016/j.fgb.2015.04.002
Steinhauer D, Salat M, Frey R, et al. (2019) A dispensable paralog of succinate dehydrogenase subunit C mediates standing resistance towards a subclass of SDHI fungicides in Zymoseptoria tritici. PLoS Pathog 15(12): e1007780. doi: 10.1371/journal.ppat.1007780
Suemoto H, Matsuzaki Y, Iwahashi F (2019) Metyltetraprole, a novel putative complex III inhibitor, targets known QoI-resistant strains of Zymoseptoria tritici and Pyrenophora teres. Pest Management Science 75: 1181-1189. doi: 10.1002/ps.5288
Suffert F, Delestre G, Gélisse S (2018) Sexual reproduction in the fungal foliar pathogen Zymoseptoria tritici is driven by antagonistic density-dependence mechanisms. bioRxiv 290072. doi: 10.1101/290072
Sykes EM, Sackett KE, Severns PM, Mundt CC (2018) Sensitivity variation and cross-resistance of Zymoseptoria tritici to azole fungicides in North America. European Journal of Plant Pathology 151(1): 269–274. doi: 10.1007/s10658-017-1370-y
, , , et al. (2021) Three LysM effectors of Zymoseptoria tritici collectively disarm chitin‐triggered plant immunity. Mol Plant Pathology 22: 683– 693. doi: 10.1111/mpp.13055
Tiley AMM, White HJ, Foster GD, Bailey AM (2019) The ZtvelB Gene Is Required for Vegetative Growth and Sporulation in the Wheat Pathogen Zymoseptoria tritici. Front. Microbiol. 10: 2210. doi: 10.3389/fmicb.2019.02210
Torriani SF, Brunner PC, McDonald BA, Sierotzki H (2009) QoI resistance emerged independently at least 4 times in European populations of Mycosphaerella graminicola. Pest Manag. Sci. 65: 155–62. doi: 10.1002/ps.1662
Torriani SFF, Melichar JPE, Mills C, et al. (2015) Zymoseptoria tritici: a major threat to wheat production, integrated approaches to control. Fungal Genetics and Biology 79: 8-12. doi: 10.1016/j.fgb.2015.04.010
van den Bosch F, Smith J, Wright P, et al. (2021) Maximizing realized yield by breeding for disease tolerance: A case study for Septoria tritici blotch. Plant Pathology 00: 1– 9. doi: 10.1111/ppa.13509
Vidal T, Lusley P, Leconte M, et al. (2017) Cultivar architecture modulates spore dispersal by rain splash: A new perspective to reduce disease progression in cultivar mixtures. PLoS ONE 12(11): e0187788. doi: 10.1371/journal.pone.0187788
, , , The identification of a transposon affecting the asexual reproduction of the wheat pathogen Zymoseptoria tritici. Mol Plant Pathol. 22: 800– 816. doi: 10.1111/mpp.13064
Yamashita M, Fraaije B (2018) Non-target site SDHI resistance is present as standing genetic variation in field populations of Zymoseptoria tritici. Pest Manag Sci. 74(3): 672-681. doi: 10.1002/ps.4761
Yang L, Gao F, Shang L, Zhan J, McDonald BA (2013) Association between Virulence and Triazole Tolerance in the Phytopathogenic Fungus Mycosphaerella graminicola. PLoS ONE 8(3): e59568. doi: 10.1371/journal.pone.0059568
Yang N, McDonald MC, Solomon PS, et al. (2018) Genetic mapping of Stb19, a new resistance gene to Zymoseptoria tritici in wheat. Theoretical and Applied Genetics 1–9. doi: 10.1007/s00122-018-3189-0
Yao C, Meyer KG, Gallup C, etc. (2021) Florylpicoxamid, a new picolinamide fungicide with broad spectrum activity. Pest Manag Sci. doi: 10.1002/ps.6483
Yemelin A, Brauchler A, Jacob S, et al. (2017) Identification of factors involved in dimorphism and pathogenicity of Zymoseptoria tritici. PLoS ONE12(8): e0183065. doi: 10.1371/journal.pone.0183065
Zhan J, Stefanato FL, McDonald BA (2006) Selection for increased cyproconazole tolerance in Mycosphaerella graminicola through local adaptation and in response to host resistance. Mol Plant Pathol. 7(4): 259-68. doi: 10.1111/j.1364-3703.2006.00336.x
Zhang Z, Running KL, Seneviratne S, et al. (2021) A protein kinase‐major sperm protein gene hijacked by a necrotrophic fungal pathogen triggers disease susceptibility in wheat. The Plant Journal. Accepted. doi: 10.1111/tpj.15194
Zhong Z, Marcel TC, Hartmann FE, et al. (2017) A small secreted protein in Zymoseptoria tritici is responsible for avirulence on wheat cultivars carrying the Stb6 resistance gene. New Phytologist 214: 619–631. doi: 10.1111/nph.14434
Artículos
Another blow to fungal infection. ROTHAMSTED RESEARCH, Frebrero 12, 2018