https://doi.org/10.1094/PHYTO-98-6-0632.
Condición fitosanitaria: Presente
Grupo de cultivos: Cereales
Rango de hospedantes: muy específico / estrecho
Hospedante primario o agronómico (estado uredinial/telial): trigo blando o común (Triticum aestivum L.), trigo duro (T. turgidum var. durum L.), trigo emmer cultivado (T. dicoccum Schrank), trigo emmer silvestre (T. dicoccoides Korn) y triticale (Triticosecale). Pst puede infectar ciertas cebadas cultivadas (Hordeum vulgare L.) y centeno (Secale cereale L.), pero generalmente no causa epidemias graves. Además, Pst puede infectar especies de pastos naturalizados y mejorados, como Elymus canadensis L., Leymus secalinus Hochst, Agropyron spp. Garetn, Hordeum spp. L., Phalaris spp. L y Bromus unioloides Kunth. (estadios telial/uredinial) (Chen et al., 2014).
Hospedante intermediario (estado pycnial/aecial): Berberis o agracejo (Berberis chinensis, B. koreana, B. holstii, B. vulgaris, B. shensiana, B. potaninii, B. dolichobotrys, B. heteropoda, etc.) y uva de Oregon (Mahonia aquifolium, Mahonia spp., etc.) (estadios pycnial / aecial) (Chen et al., 2014).
Epidemiología: policíclica, subaguda
Ciclo: macrocíclica, heteroica
Etiología: Hongo. Biotrófico
Agente causal: Puccinia striiformis f. sp. tritici Westend., (1854) (Pst)
Taxonomía: Fungi > Basidiomycota > Pucciniomycotina > Pucciniomycetes > Pucciniales > Pucciniaceae > Puccinia
.
.
.
Nuevas razas detectadas en Argentina:
SEVERE EPIDEMICS OF WHEAT YELLOW RUST IN ARGENTINA. GRRC. 09-02-2018
GRRC Summary of Puccinia striiformis race analysis 2017 (online February 10, 2018)
.
.
Antecedentes
Históricamente, P. striiformis siempre se manifestó con mayor frecuencia en la región sur de la provincia de Buenos Aires, debido a sus requerimientos de temperaturas frescas. Sin embargo, en las últimas campañas agrícolas, desde 2017/2018 y 2018/2019, se han desarrollado epidemias inéditas en zonas de mayores temperaturas medias, como la zona núcleo de la Pradera Pampeana (sur de Santa Fe, norte de Buenos Aires, Córdoba y Entre Ríos), y desde Agosto 2018 se ha detectado en la provincia de Tucumán.
Un caso similar de adaptación de Pst a regiones con temperaturas medias más cálidas había sido previamente ya observado en el sur de E.E.U.U. De acuerdo con las investigaciones de Milus et al. (2006, 2009), los nuevos aislamientos estaban mejor adaptados y, por lo tanto, fueron más agresivos a temperaturas más altas que los antiguos aislamientos. Estas diferencias pueden haber contribuido a la gravedad de las epidemias registradas en esa región y al rango geográfico de expansión para la roya amarilla en USA (Markell y Milus, 2008). Los nuevos aislamientos terminaron por reemplazar a los viejos, al igual que ocurrió más recientemente en Europa. En E.E.U.U. también se ha comprobado que Pst es capaz de acumular diferentes genes de virulencia para producir razas más complejas (Liu et al., 2017). En ese país se detectaron más razas, razas más nuevas y razas con más genes de virulencia desde el año 2000 que antes de 2000. En comparación con la población de 1968 en E.E.U.U., en la que cada raza tenía un número medio de un gen de virulencia, la población de 2009 tenía razas que poseían cada una más de 10 genes de virulencia en promedio. Recientemente en China también se comprobó que Pst posee una alta diversidad y recombinación genética (Duan et al., 2010).
.
Síntomas y signos
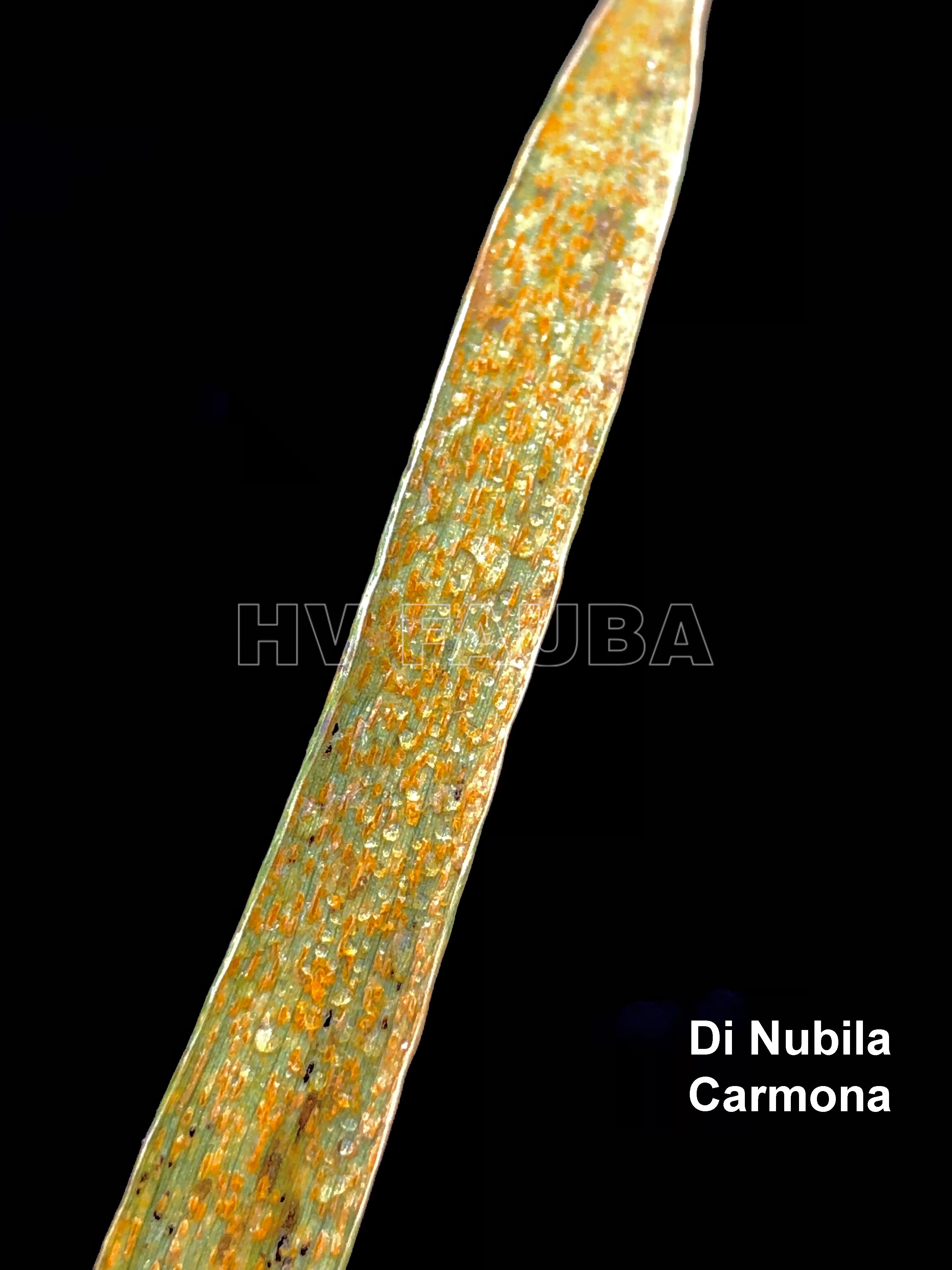
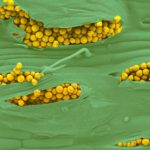
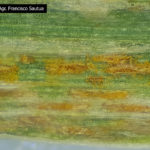
Las pústulas amarillentas ocurren sobre las hojas y están dispuestas en estrías paralelas a las nervaduras.
.
- Autores: Silvana Di Nubila, Marcelo Carmona
- Autores: Silvana Di Nubila, Marcelo Carmona
- Autor: Yue Jin
- Roya amarilla en DM Ceibo, Bigand, Sta Fe. Autores: Silvana Di Nubila, Marcelo Carmona
- Roya amarilla en DM Ceibo, Bigand, Sta Fe. Autores: Silvana Di Nubila, Marcelo Carmona
- Roya amarilla en DM Ceibo, Bigand, Sta Fe. Autores: Silvana Di Nubila, Marcelo Carmona
- Roya amarilla en DM Ceibo, Bigand, Sta Fe. Autores: Silvana Di Nubila, Marcelo Carmona
.
.
.
.
Condiciones predisponentes
La capacidad de esporulación y la eficiencia de infección de P. striiformis se ven afectadas principalmente por la temperatura del aire, la duración del mojado foliar (horas) y la intensidad de la luz (de Vallavieille-Pope et al., 1995). El efecto combinado de las variables climáticas que conducen a la infección por P. striiformis y el posterior progreso a campo no se ha establecido completamente, aún se requiere más investigación con las nuevas cepas adaptadas a mayores temperaturas.
* Para el establecimiento de la enfermedad (infecciones primarias):
la formación de rocío durante varias horas es una condición predisponente ya que las uredosporas de Pst necesitan al menos 3 horas con valores cercanos a la saturación, para germinar e infectar. Las lluvias pueden ser predisponentes porque en general aseguran las horas de mojado foliar. Las lluvias podrían contribuir a la dispersión de las uredinosporas, pero lluvias en exceso o intensas podrían remover esporas suspendidas en el aire, disminuyendo las existencias de esporas disponibles (stock) e inhibir procesos de esporulación durante horas (Geagea et al., 1999; 2000).
Los requisitos de temperatura del aire para las distintas fases de desarrollo, desde la germinación hasta la esporulación, difieren ligeramente. Estudios previos (con las razas históricas no adaptadas a mayores temperaturas) determinaron temperaturas con óptimos que oscilan entre 10 y 15 °C, y máximos alrededor de 20 °C (Sache, 2000). En Francia, Mboup et al. (2012) estudiaron la adaptación específica a la temperatura de Pst. Mediante experimentos de laboratorio, estudiaron aislados de Pst del norte (más frío) y sur (más cálido) de Francia en cuanto a su capacidad para germinar e infectar cultivares de trigo harinero y duro en un gradiente de temperatura (se determinó la capacidad de infección con pruebas de patogenicidad a las temperaturas 7, 10, 15, 20 y 25°C). Las interacciones del origen del patógeno × temperatura para la infectividad y la tasa de germinación sugieren una adaptación local a los regímenes de alta y baja temperatura en el sur y el norte. Los experimentos de competencia en los sitios de campo del sur y del norte mostraron una ventaja competitiva general de los aislamientos del sur sobre los del norte. Esta ventaja fue particularmente pronunciada en el sur, de acuerdo con un modelo que integra la infectividad del laboratorio y la variación de la temperatura de campo. La estructura estable de la población de Pst en Francia probablemente refleja la adaptación a factores ecológicos y genéticos: la persistencia de las cepas de Pst del sur puede deberse a la adaptación al clima mediterráneo más cálido; y la persistencia de las cepas de Pst del norte puede explicarse por la adaptación a los cultivares de uso común, para los cuales los aislamientos del sur carecen de los genes de virulencia relevantes. En Luxemburgo, Europa, El Jarroudi et al. (2017) encontraron que una combinación de humedad relativa >92% y temperaturas entre 4°C y 16°C durante un mínimo de 4 horas continuas, asociado con lluvias ≤ 0.1 mm, fue óptima para el desarrollo de epidemias de roya amarilla o estriada del trigo.
En Argentina, históricamente, temperaturas entre 10 a 15 °C fueron las conducentes para las cepas de Pst endémicas en el sudeste de Buenos Aires. Esta es la razón por la cual la ocurrencia epidemiológica de la roya amarilla, durante los últimos años, fue siempre esporádica y recluida a regiones con temperaturas medias más bajas, como el sudesde de la provincia de Buenos Aires. Sin embargo, en las últimas campañas agrícolas, desde 2017/2018 y 2018/2019, se han desarrollado epidemias inéditas en zonas de mayores temperaturas medias, como la zona núcleo de la Pradera Pampeana (sur de Santa Fe, norte de Buenos Aires, Córdoba y Entre Ríos), y desde agosto 2018 se ha detectado en la provincia de Tucumán.
.
* Para la dispersión de la enfermedad (infecciones secundarias): Días soleados y ventosos y noches con formación de rocío durante varias horas.
.
En inoculaciones artificiales en Canadá, se observaron aumentos significativos en los niveles de severidad luego de la aplicación de 1.2 x 107 esporas. En conjunto, estos resultados demostraron que la severidad de la roya lineal aumentó con el aumento de la concentración de esporas solo en niveles altos de esporas.
.
.
Ciclo de la enfermedad
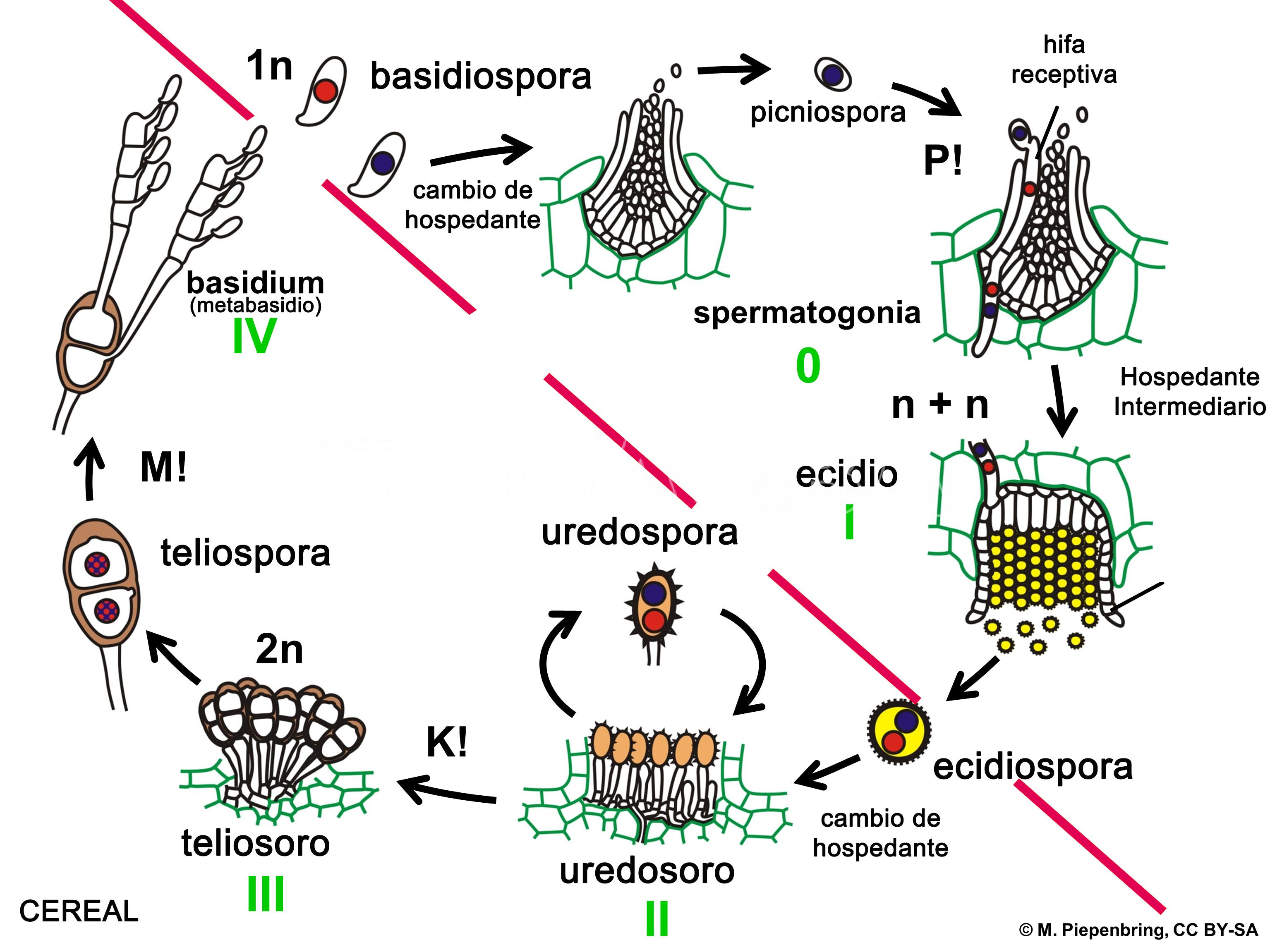
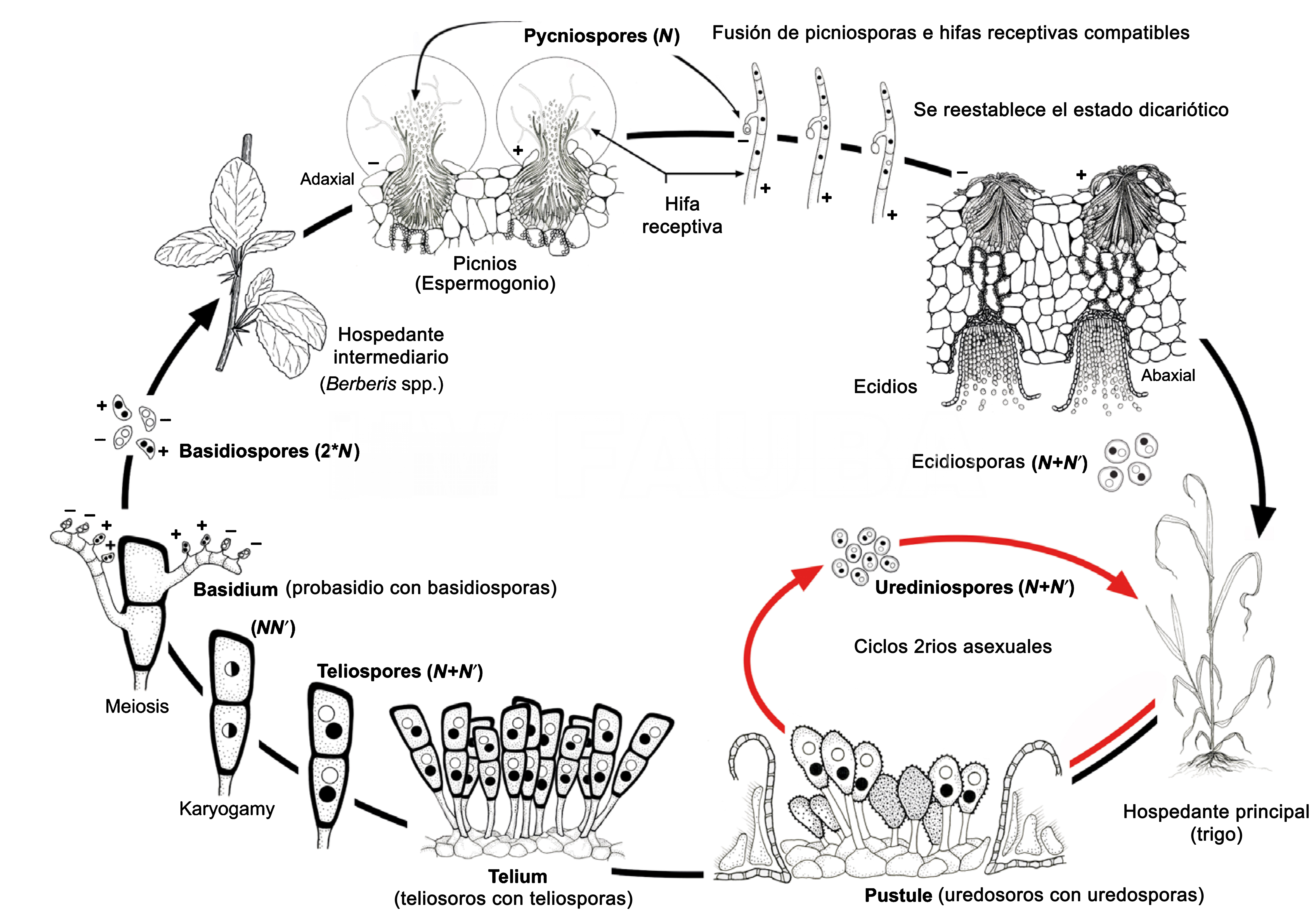
Se ha demostrado recientemente que la roya estriada del trigo, causada por Pst, es heteroica y macrocíclica (Jin et al., 2010; Zhao et al., 2013).
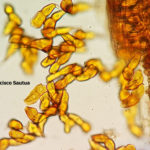
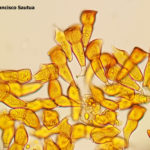
Las basidiosporas binucleadas, doblemente haploides (2*N), de corta duración, infectan el agracejo (Berberis spp) al comienzo del ciclo de infección sexual (ver figuras a continuación). Durante las infecciones exitosas, Pst forma picnios en la cara superior (adaxial) de la hoja y produce picniosporas haploides mononucleadas (N), con tipo de apareamiento específico. La fusión de picniosporas con hifas receptivas de un picnio compatible con el tipo de apareamiento inicia la dicariotización y el desarrollo de un ecidio (aecium) (N+N′) en la cara inferior (abaxial) de la hoja. Múltiples eventos distintos de dicariotización pueden ocurrir dentro de un solo picnio, dando lugar a ecidios (aecias) genéticamente diversos. Las ecidiosporas (aeciosporas) vegetativas (N+N′) solo pueden infectar al hospedante primario o agronómico (trigo), e iniciar el ciclo de infección asexual. La infección exitosa del trigo conduce a la formación de pústulas uredosóricas de color amarillo en ambos lados de la hoja, y cada pústula expulsa miles de urediniosporas dicarióticas (N+N′). Para completar su ciclo de vida sexual, Pst cambia a la formación de telios (teliosoros) al final de la temporada de crecimiento del trigo y produce teliosporas inicialmente dicarióticas (N+N′) de paredes gruesas. Luego, estas esporas sufren fusión nuclear (cariogamia, NN′) y sucesivas meiosis con recombinación sexual generando nueva diversidad genética. Luego, las esporas germinan inmediatamente, iniciando el desarrollo del (meta o pro)basidio y la producción de cuatro basidiosporas doble haploides binucleadas (2*N) listas para infectar el agracejo (hospedante intermediario).
.
- Representación esquemática del ciclo de una roya de cereal heteroica macrocíclica, como por ejemplo Puccinia striiformis f. sp. tritici. P! = plasmogamia; K! = cariogamia; M! = meiosis; 2n = células diploides; 1n = células haploides; n + n = células dicarióticas.
- Representación esquemática del ciclo de vida de Puccinia striformis f. sp. tritici, agente causal de la roya amarilla o estriada o lineal del trigo. Fuente: Schwessinger, 2016
.
.
Manejo Integrado
* Siembra de cultivares resistentes
* Uso de fungicidas de acuerdo con umbrales de decisión económica
.
.
- Uredosoros y teliosoros de Pst sobre hojas, tallos y espiguillas. Fotos tomadas en Túnez. Autor: Frederic Suffert
.
.
- 01 Uredosoros con uredosporas de roya amarilla (Pst). Variedad Algarrobo, localidad Venado Tuerto. Autor: Silvana Di Nubila.
- 03 Uredosoros con uredosporas de roya amarilla (Pst). Variedad Algarrobo, localidad Venado Tuerto. Autor: Silvana Di Nubila.
- 07 Uredosoros con uredosporas de roya amarilla (Pst). Variedad Algarrobo, localidad Venado Tuerto. Autor: Silvana Di Nubila.
- 08 Uredosoros con uredosporas de roya amarilla (Pst). Variedad Algarrobo, localidad Venado Tuerto. Autor: Silvana Di Nubila.
- 09 Uredosoros con uredosporas de roya amarilla (Pst). Variedad Algarrobo, localidad Venado Tuerto. Autor: Silvana Di Nubila.
- 11 Uredosoros con uredosporas de roya amarilla (Pst). Variedad Algarrobo, localidad Venado Tuerto. Autor: Silvana Di Nubila.
- 12 Uredosoros con uredosporas de roya amarilla (Pst). Variedad Algarrobo, localidad Venado Tuerto. Autor: Silvana Di Nubila.
- 14 Uredosoros con uredosporas de roya amarilla (Pst). Variedad Algarrobo, localidad Venado Tuerto. Autor: Silvana Di Nubila.
- 17 Uredosoros con uredosporas de roya amarilla (Pst). Variedad Algarrobo, localidad Venado Tuerto. Autor: Silvana Di Nubila.
- 18 Uredosoros con uredosporas de roya amarilla (Pst). Variedad Algarrobo, localidad Venado Tuerto. Autor: Silvana Di Nubila.
- 19 Uredosoros con uredosporas de roya amarilla (Pst). Variedad Algarrobo, localidad Venado Tuerto. Autor: Silvana Di Nubila.
- 20 Uredosoros con uredosporas de roya amarilla (Pst). Variedad Algarrobo, localidad Venado Tuerto. Autor: Silvana Di Nubila.
- 22 Gota de agua dispersando uredinosporas
- 01 Uredosoros con uredosporas de Pst en hojas de trigo. Fontezuela, Bs. As., 2017/2018, variedad DM Algarrobo.
- 05 Uredosoros con uredosporas de Pst en hojas de trigo. Fontezuela, Bs. As., 2017/2018, variedad DM Algarrobo.
- 06 Uredosoros con uredosporas de Pst en hojas de trigo. Fontezuela, Bs. As., 2017/2018, variedad DM Algarrobo.
- 08 Uredosoros con uredosporas de Pst en hojas de trigo. Fontezuela, Bs. As., 2017/2018, variedad DM Algarrobo.
- 41 Uredosporas de Pst. Fontezuela, Bs. As., 2017/2018, variedad DM Algarrobo.
- 47 Uredosporas de Pst. Fontezuela, Bs. As., 2017/2018, variedad DM Algarrobo.
- 48 Uredosporas de Pst. Fontezuela, Bs. As., 2017/2018, variedad DM Algarrobo.
- 51 Uredosporas de Pst. Fontezuela, Bs. As., 2017/2018, variedad DM Algarrobo.
- 67 Uredosporas de Pst. Fontezuela, Bs. As., 2017/2018, variedad DM Algarrobo.
- 72 Uredosporas de Pst. Fontezuela, Bs. As., 2017/2018, variedad DM Algarrobo.
- 74 Uredosporas de Pst. Fontezuela, Bs. As., 2017/2018, variedad DM Algarrobo.
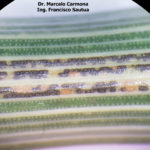
- 75 Uredosoros y teleutosoros de Pst en glumas de trigo. Fontezuela, Bs. As., 2017/2018, variedad DM Algarrobo.
- 76 Uredosoros y teleutosoros de Pst en glumas de trigo. Fontezuela, Bs. As., 2017/2018, variedad DM Algarrobo.
- 77 Teleutosoros de Pst en vainas de trigo. Fontezuela, Bs. As., 2017/2018, variedad DM Algarrobo.
- 78 Teleutosoros de Pst en vainas de trigo. Fontezuela, Bs. As., 2017/2018, variedad DM Algarrobo.
- 85 Teleutosoros de Pst en vainas de trigo. Fontezuela, Bs. As., 2017/2018, variedad DM Algarrobo.
- 86 Mycodiplosis spp alimentándose de uredinosporas de Pst en hojas de trigo (Insect mycophagy). Fontezuela, Bs. As., 2017/2018, variedad DM Algarrobo.
- 88 Mycodiplosis spp alimentándose de uredinosporas de Pst en hojas de trigo (Insect mycophagy). Fontezuela, Bs. As., 2017/2018, variedad DM Algarrobo.
- 04 Hoja de trigo con teleutosoros o teliosoros de Pst
- 01 Teliosoros de Puccinia striiformis f. sp. tritici (Pst)
- 11 Teliosporas de Pst
- 35 Teliosporas de Pst
- 07 Teliosporas de Pst
- 25 Teliosporas de Pst
- 13 Teliosporas de Pst
- 01 Uredinosporas de Puccinia striiformis f. sp. tritici (Pst) sobre rueda de tractor, luego de realizar labores en el lote. Localidad Los Cisnes, La Carlota, Cba. Variedad Klein Serpiente, campaña 2017/2018. Autor: Ing. Agr. Juan Pablo Ioele
- Pústulas con uredinosporas de Pst. Variedad DM Fuste, localidad Landeta, Sta Fe, Campaña 2017/2018. Autor: Ing. Carlos Grosso
- Izq Variedad resistente y Der Variedad susceptible a Pst. localidad Landeta, Sta Fe, Campaña 2017/2018. Autor: Ing. Carlos Grosso
- 01 Testigo sin control químico, Venado Tuerto 2017/2018, Autor: Jonatan Damiani
- 01 Tratado (con control químico), Venado Tuerto 2017/2018, Autor: Jonatan Damiani
- Bordura de trigo DM Algarrobo severamente afectada por Roya Amarilla. Fontezuela, 2017/2018
- Hoja bandera de trigo DM Algarrobo severamente afectada por Roya Amarilla. Fontezuela, 2017/2018
- Hoja bandera de trigo DM Algarrobo severamente afectada por Roya Amarilla. Fontezuela, 2017/2018
- The Sainsbury Laboratory ©
- Hoja de trigo con síntomas de roya amarilla, causada por Puccinia striiformis, creciendo en un terreno experimental en el Centro Nacional de Investigación Agrícola de Pakistán (NARC) en Islamabad, fotografiado después de la lluvia. Autor: A. Yaqub / CIMMYT
- Hoja de trigo infectada con roya amarilla (Puccinia striiformis). Las pústulas contienen urediosporas de color amarillo a naranja y generalmente forman rayas o estrías en las hojas. Las pústulas de color negro corresponden a los teleutosoros, que contienen teliosporas. Autor: Thomas Lumpkin / CIMMYT.
- 01 Pústulas de Roya Amarilla, zona de Cañuelas, campaña 2017/2018, variedad Algarrobo
- 02 Plantas de trigo infectadas con Roya Amarilla, zona de Cañuelas, campaña 2017/2018, variedad Algarrobo
- 03 Plantas de trigo infectadas con Roya Amarilla, zona de Cañuelas, campaña 2017/2018, variedad Algarrobo
- 04 Plantas de trigo infectadas con Roya Amarilla, zona de Cañuelas, campaña 2017/2018, variedad Algarrobo
- 04a Hoja de trigo con abundante esporulación de Pst, Cañuelas 2017/18, Algarrobo
- 04b Hoja de trigo con abundante esporulación de Pst, Cañuelas 2017/18, Algarrobo
- 05 Síntomas y Uredinosoros de Pst, zona Cañuelas, campaña 2017/2018, variedad Algarrobo
- 06 Síntomas y Uredinosoros de Pst, zona Cañuelas, campaña 2017/2018, variedad Algarrobo
- 07 Síntomas y Uredinosoros de Pst, zona Cañuelas, campaña 2017/2018, variedad Algarrobo
- 01 Roya amarilla controlada con aplicación de fungicida
- 02 Roya amarilla controlada con aplicación de fungicida
- 03 Roya amarilla controlada con aplicación de fungicida
- 04 Roya amarilla controlada con aplicación de fungicida
- 05 Roya amarilla controlada con aplicación de fungicida
- 06 Roya amarilla controlada con aplicación de fungicida
- Roya amarilla controlada con fungicida. Fontenzuela, 2017/2018, variedad DM Algarrobo
- Roya amarilla controlada con fungicida. Fontenzuela, 2017/2018, variedad DM Algarrobo
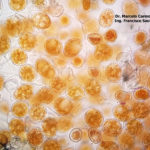
- 01 Síntomas y signos de Roya Amarilla en cultivos de trigo, Miramar, Bs As, 2017/2018. Autor: Dr. Marcelo Carmona.
- 02 Síntomas y signos de Roya Amarilla en cultivos de trigo, Miramar, Bs As, 2017/2018. Autor: Dr. Marcelo Carmona.
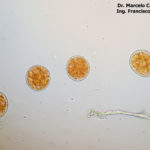
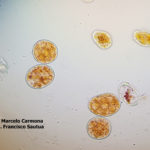
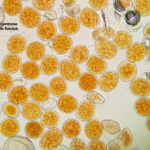
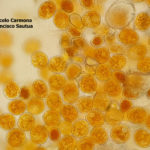
- 03 Síntomas y signos (uredinosporas) de Roya Amarilla en hoja de trigo, Miramar, Bs As, 2017/2018. Autor: Dr. Marcelo Carmona.
- 04 Síntomas y signos de Roya Amarilla en cultivos de trigo, Miramar, Bs As, 2017/2018. Autor: Dr. Marcelo Carmona.
- 05 Síntomas y signos de Roya Amarilla en cultivos de trigo, Miramar, Bs As, 2017/2018. Autor: Dr. Marcelo Carmona.
- 06 Necrosis por Roya Amarilla, Miramar, Bs As, 2017/2018. Autor: Dr. Marcelo Carmona.
- 07 Necrosis por Roya Amarilla, Miramar, Bs As, 2017/2018. Autor: Dr. Marcelo Carmona.
- 08 Necrosis por Roya Amarilla, Miramar, Bs As, 2017/2018. Autor: Dr. Marcelo Carmona.
- 09 Necrosis por Roya Amarilla, Miramar, Bs As, 2017/2018. Autor: Dr. Marcelo Carmona.
- 10 Síntomas y signos de Roya Amarilla en espigas de trigo, Miramar, Bs As, 2017/2018. Autor: Dr. Marcelo Carmona.
- 11 Síntomas y signos de Roya Amarilla en espigas de trigo, Miramar, Bs As, 2017/2018. Autor: Dr. Marcelo Carmona.
- 12 Síntomas y signos de Roya Amarilla en espigas de trigo, Miramar, Bs As, 2017/2018. Autor: Dr. Marcelo Carmona.
- 01 Pústulas de Pst en espigas de trigo, variedad DM Fuste. Oro Verde, Paraná, Entre Ríos, 2017. Autor: Ing. Agr. Norma Formento.
- 02 Pústulas de Pst en espigas de trigo, variedad DM Fuste. Oro Verde, Paraná, Entre Ríos, 2017. Autor: Ing. Agr. Norma Formento.
- 03 Pústulas de Pst en espigas de trigo, variedad DM Fuste. Oro Verde, Paraná, Entre Ríos, 2017. Autor: Ing. Agr. Norma Formento.
- 04 Pústulas de Pst en espigas de trigo, variedad DM Fuste. Oro Verde, Paraná, Entre Ríos, 2017. Autor: Ing. Agr. Norma Formento.
- 05 Pústulas de Pst en espigas de trigo, variedad DM Fuste. Oro Verde, Paraná, Entre Ríos, 2017. Autor: Ing. Agr. Norma Formento.
Detecciones 2018:
- 01 Primeras detecciones de Roya amarilla del trigo en Agosto 2018, z31-z32. Autor: Ing. Norma Formento, INTA Paraná, Entre Ríos.
- 02 Primeras detecciones de Roya amarilla del trigo en Agosto 2018, z31-z32. Autor: Ing. Norma Formento, INTA Paraná, Entre Ríos.
- 01 Deteccion de Roya amarilla en Maggiolo, Sta Fe, variedades de trigo DM Ceibo y DM Algarrobo. Autor: Ing. Jonatan Damiani.
- 02 Deteccion de Roya amarilla en Maggiolo, Sta Fe, variedades de trigo DM Ceibo y DM Algarrobo. Autor: Ing. Jonatan Damiani.
- 03 Deteccion de Roya amarilla en Sancti Spiritu, Sta Fe, variedades de trigo DM Ceibo y DM Algarrobo. Autor: Ing. Jonatan Damiani.
- 04 Deteccion de Roya amarilla en Sancti Spiritu, Sta Fe, variedades de trigo DM Ceibo y DM Algarrobo. Autor: Ing. Jonatan Damiani.
- 05 Deteccion de Roya amarilla en Sancti Spiritu, Sta Fe, variedades de trigo DM Ceibo y DM Algarrobo. Autor: Ing. Jonatan Damiani.
- 06 Deteccion de Roya amarilla en Sta Fe, variedades de trigo DM Ceibo y DM Algarrobo. Autor: Ing. Jonatan Damiani.
- 07 Deteccion de Roya amarilla en Sta Fe, variedades de trigo DM Ceibo y DM Algarrobo. Autor: Ing. Jonatan Damiani.
- 08 Deteccion de Roya amarilla en Sta Fe, variedades de trigo DM Ceibo y DM Algarrobo. Autor: Ing. Jonatan Damiani.
- 01 Detección de Roya amarilla en Bahia Blanca, variedad ACA303, sembrada a mediados de mayo. Autor: Ing. Ana Storm, CEI Barrow INTA
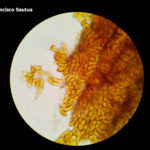
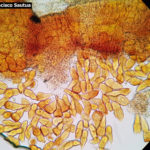
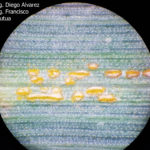
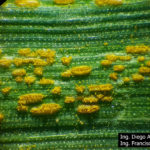
- 01 Uredosoros con uredosporas de P. striiformis f. sp. tritici en hoja de trigo variedad DM Algarrobo, Pergamino, Bs As, z39, 01 Octubre 2018. Autores: Ing. Diego Alvarez, Ing. Francisco Sautua, FAUBA.
- 02 Uredosoros con uredosporas de P. striiformis f. sp. tritici en hoja de trigo variedad DM Algarrobo, Pergamino, Bs As, z39, 01 Octubre 2018. Autores: Ing. Diego Alvarez, Ing. Francisco Sautua, FAUBA.
- 03 Uredosoros con uredosporas de P. striiformis f. sp. tritici en hoja de trigo variedad DM Algarrobo, Pergamino, Bs As, z39, 01 Octubre 2018. Autores: Ing. Diego Alvarez, Ing. Francisco Sautua, FAUBA.
- 04 Uredosoros con uredosporas de P. striiformis f. sp. tritici en hoja de trigo variedad DM Algarrobo, Pergamino, Bs As, z39, 01 Octubre 2018. Autores: Ing. Diego Alvarez, Ing. Francisco Sautua, FAUBA.
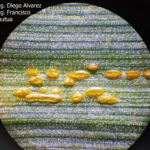
- 05 Uredosoros con uredosporas de P. striiformis f. sp. tritici en hoja de trigo variedad DM Algarrobo, Pergamino, Bs As, z39, 01 Octubre 2018. Autores: Ing. Diego Alvarez, Ing. Francisco Sautua, FAUBA.
- 06 Uredosoros con uredosporas de P. striiformis f. sp. tritici en hoja de trigo variedad DM Algarrobo, Pergamino, Bs As, z39, 01 Octubre 2018. Autores: Ing. Diego Alvarez, Ing. Francisco Sautua, FAUBA.
- 07 Uredosoros con uredosporas de P. striiformis f. sp. tritici en hoja de trigo variedad DM Algarrobo, Pergamino, Bs As, z39, 01 Octubre 2018. Autores: Ing. Diego Alvarez, Ing. Francisco Sautua, FAUBA.
- 08 Uredosoros con uredosporas de P. striiformis f. sp. tritici en hoja de trigo variedad DM Algarrobo, Pergamino, Bs As, z39, 01 Octubre 2018. Autores: Ing. Diego Alvarez, Ing. Francisco Sautua, FAUBA.
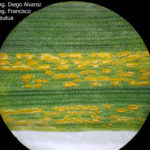
- 09 Uredosoros con uredosporas de P. striiformis f. sp. tritici en hoja de trigo variedad DM Algarrobo, Pergamino, Bs As, z39, 01 Octubre 2018. Autores: Ing. Diego Alvarez, Ing. Francisco Sautua, FAUBA.
- 10 Uredosoros con uredosporas de P. striiformis f. sp. tritici en hoja de trigo variedad DM Algarrobo, Pergamino, Bs As, z39, 01 Octubre 2018. Autores: Ing. Diego Alvarez, Ing. Francisco Sautua, FAUBA.
- 11 Uredosoros con uredosporas de P. striiformis f. sp. tritici en hoja de trigo variedad DM Algarrobo, Pergamino, Bs As, z39, 01 Octubre 2018. Autores: Ing. Diego Alvarez, Ing. Francisco Sautua, FAUBA.
- 12 Uredosoros con uredosporas de P. striiformis f. sp. tritici en hoja de trigo variedad DM Algarrobo, Pergamino, Bs As, z39, 01 Octubre 2018. Autores: Ing. Diego Alvarez, Ing. Francisco Sautua, FAUBA.
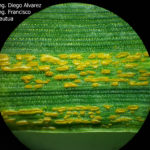
- 13 Uredosoros con uredosporas de P. striiformis f. sp. tritici en hoja de trigo variedad DM Algarrobo, Pergamino, Bs As, z39, 01 Octubre 2018. Autores: Ing. Diego Alvarez, Ing. Francisco Sautua, FAUBA.
- 14 Uredosoros con uredosporas de P. striiformis f. sp. tritici en hoja de trigo variedad DM Algarrobo, Pergamino, Bs As, z39, 01 Octubre 2018. Autores: Ing. Diego Alvarez, Ing. Francisco Sautua, FAUBA.
- 01 Roya amarilla o lineal en trigo candeal Autor: Ing. Agr. Liliana Wehrhahne, Chacra Experimental Integrada Barrow INTA.
- 01 Uredinosporas de Roya Amarilla adheridas a ropa de monitoreador. Variedad DM Algarrobo, en Ameghino. Autor: Ing. Mauro Montarini.
- 02 01 Uredinosporas de Roya Amarilla adheridas a zapatos de monitoreador. Variedad DM Algarrobo, en Ameghino. Autor: Ing. Mauro Montarini.
- 01 Zapato de monitoreador completamente cubierto por uredinosporas de Pst. Autor: Ing. Agr. Heber Adami (Carlos Casares).
- 02 Zapato de monitoreador completamente cubierto por uredinosporas de Pst. Autor: Ing. Agr. Heber Adami (Carlos Casares).
- 01 Bota de monitoreador repleta de uredinosporas de Pst. Autor: Ing. Agr. Patricio Martinez, Arenasa.
- 02 Bota de monitoreador repleta de uredinosporas de Pst. Autor: Ing. Agr. Patricio Martinez, Arenasa.
.
- Autor: Edel Pérez-López
.
Detecciones 2019:
- Inoculo de Puccinia striiformis f sp tritici arrastrado por la lluvia. Autor: Diego Alvarez
- Uredinosporas de P. striiformis f. sp. tritici arrastradas por el agua de lluvia. Autor: Diego Alvarez
.
- Roya amarilla en Triticale. Autor: P. Stephen Baenziger
.
Detecciones 2021:
- Uredosporas de Pst en zapatos de monitoreadores, zona de 9 de Julio, Bs As. Autores: Margarita Ranzatto, Alvaro Merlo.
- Uredosporas de Pst en zapatos de monitoreadores, zona de 9 de Julio, Bs As. Autores: Margarita Ranzatto, Alvaro Merlo.
.
.
Novedades
* Nuevas razas detectadas en Argentina: SEVERE EPIDEMICS OF WHEAT YELLOW RUST IN ARGENTINA. GRRC. 09-02-2018
* Carmona M, Sautua F, Pérez-Hernández O, Grosso C, Vettorello L, Milanesio B, Corvi E, Almada G, Hovmøller M (2019) Rapid emergency response to yellow rust epidemics caused by newly introduced lineages of Puccinia striiformis f. sp. tritici in Argentina. Tropical Plant Pathology 44: 385–391. doi: 10.1007/s40858-019-00295-y
* GRRC Summary of Puccinia striiformis race analysis 2017 (online February 10, 2018)
* Protocolo muestreo Roya Amarilla Trigo 2017 FAUBA Carmona Sautua (video)
.
.
Fotos
Stripe Rust. Washington State University
Puccinia striiformis Westend. Rust Fungi of Australia.
.
Videos
Rust: the fungi that attacks plants. Created by Chris Hammang, Producer Sean O’Donoghue, Scientific Consultant Peter Dodds. C SIRO (Video)
Stripe rust of wheat (video)
GCTV20: Rust Analysis – Stripe Rust (Australia) (video)
Triple Rust Resistance (video)
Integrated Genomics Approach to combat wheat yellow rust (video)
Yellow rust in organic Warrior winter wheat (video)
Yellow Rust Disease Destroys Wheat Crop in Northern India (video)
Stripe rust of wheat. CPS Canadian Phytopathological Society (video)
GWAS identifies QTLs for yellow rust resistance in Ethiopian durum wheat (video)
.
Notas
Stripe Rust of Wheat on the Move. ILLINOIS FIELD CROP DISEASE BLOG
.
.
.
Bibliografía
BGRI Borlaug Global Rust Initiative
Stripe Rust. Washington State University
Abou‐Attia MA, Wang X, Nashaat Al‐Attala M, et al. (2016) TaMDAR6 acts as a negative regulator of plant cell death and participates indirectly in stomatal regulation during the wheat stripe rust–fungus interaction. Physiologia Plantarum 156: 262-277. doi: 10.1111/ppl.12355
Ali S, Gladieux P, Leconte M, et al. (2014) Origin, Migration Routes and Worldwide Population Genetic Structure of the Wheat Yellow Rust Pathogen Puccinia striiformis f.sp. tritici. PLoS Pathog 10(1): e1003903. doi: 10.1371/journal.ppat.1003903
Ali, S., Leconte, M., Rahman, H. et al. (2014) A high virulence and pathotype diversity of Puccinia striiformis f.sp. tritici at its centre of diversity, the Himalayan region of Pakistan. European Journal of Plant Pathology 140(2): 275-290. doi: 10.1007/s10658-014-0461-2
Ali S, Rodriguez-Algaba J, Thach T, et al. (2017) Yellow Rust Epidemics Worldwide Were Caused by Pathogen Races from Divergent Genetic Lineages. Frontiers in Plant Science 8: 1057. doi: 10.3389/fpls.2017.01057
Ali S, Sharma S, Leconte M, et al. (2017) Low pathotype diversity in a recombinant Puccinia striiformis population through convergent selection at the eastern Himalayan centre of diversity (Nepal). Plant Pathology (in press). doi: 10.1111/ppa.12796
An T, Cai Y, Zhao S, et al. (2016) Brachypodium distachyon T-DNA insertion lines: a model pathosystem to study nonhost resistance to wheat stripe rust. Scientific Reports 6: 25510. doi: 10.1038/srep25510
Anikster Y (1983) Binucleate basidiospores – a general rule in rust fungi. Transactions of the British Mycological Society 81: 624 626.
Anikster Y, Eilam T, Bushnell WR, Kosman E (2005) Spore dimensions of Puccinia species of cereal hosts as determined by image analysis. Mycologia 97(2): 474-484. doi: 10.1080/15572536.2006.11832823
(2020) Detection and quantification of airborne spores from six important wheat fungal pathogens in southern Alberta. Canadian Journal of Plant Pathology, doi: 10.1080/07060661.2020.1817795
Araujo GT, Gaudet DA, Amundsen E, et al. (2023) Inoculum threshold for stripe rust infection in wheat. Canadian Journal of Plant Pathology. doi: 10.1080/07060661.2023.2177888
Arora S, Kaur S, Dhillon GS, et al. (2021) Introgression and genetic mapping of leaf rust and stripe rust resistance in Aegilops triuncialis. J Genet 100: 6. doi: 10.1007/s12041-020-01253-3
Asaturova AM, Zhevnova NA, Tomashevich NS, et al. (2022) Evaluation of Bacillus velezensis Biocontrol Potential against Fusarium Fungi on Winter Wheat. Agronomy 12(8): 1956. doi: 10.3390/agronomy12081956
Athiyannan N, Zhang P, McIntosh R, et al. (2022) Haplotype variants of the stripe rust resistance gene Yr28 in Aegilops tauschii. Theor Appl Genet. doi: 10.1007/s00122-022-04221-w
Atta BM, Saleem M, Bilal M, et al. (2022) Early detection of stripe rust infection in wheat using light-induced fluorescence spectroscopy. Photochem Photobiol Sci. doi: 10.1007/s43630-022-00303-2
Awaad HA, El-Naggar DR (2021) Developing Rust Resistance of Wheat Genotypes Under Egyptian Conditions. In: Awaad H., Abu-hashim M., Negm A. (eds) Mitigating Environmental Stresses for Agricultural Sustainability in Egypt. Springer Water. Springer, Cham. doi: 10.1007/978-3-030-64323-2_12
Awais M, Ali S, Ju M, et al. (2022) Countrywide inter-epidemic region migration pattern suggests the role of southwestern population in wheat stripe rust epidemics in China. Environ Microbiol. doi: 10.1111/1462-2920.16096
Aylor DE (2003) Spread of plant disease on a continental scale: role of aerial dispersal of pathogens. Ecology 84: 1989-1997. doi: 10.1890/01-0619
Babu P, Baranwal DK, Harikrishna, et al. (2020) Application of Genomics Tools in Wheat Breeding to Attain Durable Rust Resistance. Frontiers in Plant Science 11: 567147. doi: 10.3389/fpls.2020.567147
, , , et al. (2021) RNAi‐mediated stable silencing of TaCSN5 confers broad‐spectrum resistance to Puccinia striiformis f. sp. tritici. Molecular Plant Pathology 22: 410– 421. doi: 10.1111/mpp.13034
Bai Q, Wan A, Wang M, et al. (2021) Population Diversity, Dynamics, and Differentiation of Wheat Stripe Rust Pathogen Puccinia striiformis f. sp. tritici From 2010 to 2017 and Comparison With 1968 to 2009 in the United States. Front. Microbiol. 12: 696835. doi: 10.3389/fmicb.2021.696835
Bai Q, Wan A, Wang M, (2021) Molecular Characterization of Wheat Stripe Rust Pathogen (Puccinia striiformis f. sp. tritici) Collections from Nine Countries. Int J Mol Sci. 22(17):9457. doi: 10.3390/ijms22179457
Bai Q, Wang M, Xia C, et al. (2022) Identification of Secreted Protein Gene-Based SNP Markers Associated with Virulence Phenotypes of Puccinia striiformis f. sp. tritici, the Wheat Stripe Rust Pathogen. International Journal of Molecular Sciences 23(8): 4114. doi: 10.3390/ijms23084114
Bal RS (2014) Effect of some fungicides and time of fungicidal spray on stripe rust of wheat. Journal of Plant and Pest Science 1(1): 39-43.
Balint-Kurti P (2019) The plant hypersensitive response: concepts, control and consequences. Molecular Plant Pathology 20: 1163-1178. doi: 10.1111/mpp.12821
, , , et al. (2023) The interaction of two Puccinia striiformis f. sp. tritici effectors modulates high-temperature seedling-plant resistance in wheat. Molecular Plant Pathology 24: 1522–1534. doi: 10.1111/mpp.13390
Baranwal D (2022) Genetic and genomic approaches for breeding rust resistance in wheat. Euphytica 218: 159. doi: 10.1007/s10681-022-03111-y
Belay AF, Jenber AJ, Mekonnen TW (2025) Distribution and Associated Factors Influencing Yellow Rust (Puccinia striiformis f. sp. tritici) Epidemic Developments in Bread Wheat (Triticum aestivum) in Amhara Region, Ethiopia. Journal of Crop Health 77: 62 (2025). doi: 10.1007/s10343-025-01129-5
Bhardwaj SC, Singh GP, Gangwar OP, et al. (2019) Status of Wheat Rust Research and Progress in Rust Management-Indian Context. Agronomy 9 (12): 1–14. doi: 10.3390/agronomy9120892
Bhatti JA, Shahzad A, Bhatti WA, et al. (2024) A short review on the impacts of evolving and adapted yellow rust strains in wheat. Pure Appl. Biol.,13(3):328-334. doi: 10.19045/bspab.2024.130029
Bockus WW, Bowden RL, Hunger RM, Murray TD, Smiley RW, Morrill W (eds.) (2010) Compendium of Wheat Diseases and Insects, Third Edition. APS Press, Minneapolis
Bouvet L, Holdgate S, James L, et al. (2021) The evolving battle between yellow rust and wheat: implications for global food security. Theor Appl Genet. doi: 10.1007/s00122-021-03983-z
Bozkurt TO, Mcgrann GR, Maccormack R, et al. (2010) Cellular and transcriptional responses of wheat during compatible and incompatible race‐specific interactions with Puccinia striiformis f. sp. tritici. Molecular Plant Pathology 11: 625-640. doi: 10.1111/j.1364-3703.2010.00633.x
Brar GS, Graf R, Knox R, et al. (2017) Reaction of differential wheat and triticale genotypes to natural stripe rust [Puccinia striiformis f. sp. tritici] infection in Saskatchewan, Canada, Canadian Journal of Plant Pathology. doi: 10.1080/07060661.2017.1341433
Brar GS, Ali S, Qutob D, et al. (2018) Genome re-sequencing and simple sequence repeat markers reveal the existence of divergent lineages in the Canadian Puccinia striiformis f. sp. tritici population with extensive DNA methylation. Environ. Microbiol. 20: 1498-1515. doi: 10.1111/1462-2920.14067
Bueno-Sancho V, Persoons A, Hubbard A, et al. (2017) Pathogenomic analysis of wheat yellow rust lineages detects seasonal variation and host specificity. Genome Biology and Evolution evx241. doi: 10.1093/gbe/evx241
Bulli P, Zhang J, Chao S, et al. (2016) Genetic Architecture of Resistance to Stripe Rust in a Global Winter Wheat Germplasm Collection. G3 (Bethesda) 6(8): 2237-2253. doi: 10.1534/g3.116.028407
Campos P, Formento N, Couretort L, Alberione E (2016) Aparición epifítica de roya amarilla del trigo en la región pampeana argentina.
Campos PE, Cardarelli FO (2023) Situación actual de las royas de trigo en Argentina. Cambios en las poblaciones de los patógenos y comportamiento sanitario de los cultivares de trigo. INTA. Link
Cantu D, Segovia V, MacLean D, et al. (2013) Genome analyses of the wheat yellow (stripe) rust pathogen Puccinia striiformis f. sp. tritici reveal polymorphic and haustorial expressed secreted proteins as candidate effectors. BMC Genomics 14: 270. doi: 10.1186/1471-2164-14-270
Carmona (2016) Consideraciones para el monitoreo y control de la roya amarilla con fungicidas.
Carmona M, Sautua F (2017) La problemática de la resistencia de hongos a fungicidas. Causas y efectos en cultivos extensivos. Agronomía & Ambiente Rev. Facultad de Agronomía UBA 37(1): 1-19.
Carmona MA & Sautua F (2018) Epidemias de roya amarilla del trigo: nuevas razas en el mundo, monitoreo y decisión de uso de fungicidas. Agronomía y ambiente: Revista de la Facultad de Agronomía de la Universidad de Buenos Aires 38 (1): 37-58. LINK
Carmona M, Sautua F, Pérez-Hérnandez O, Reis EM (2020) Role of Fungicide Applications on the Integrated Management of Wheat Stripe Rust. Frontiers in Plant Science 11: 733. doi: 10.3389/fpls.2020.00733
Chemayek B, Bansal UK, Miah H, et al. (2021) Assessment of Genetic Diversity for Stem Rust and Stripe Rust Resistance in an International Wheat Nursery Using Phenotypic and Molecular Technologies. Uganda Journal of Agricultural Sciences 20. doi: 10.4314/ujas.v20i1.1
Chemayek B, Wagoire W, Bansal U, Bariana H (2024) A Combination of Three Genomic Regions Conditions High Level of Adult Plant Stripe Rust Resistance in Australian Wheat Cultivar Sentinel. Plants 13(1): 129. doi: 10.3390/plants13010129
Chen X, Moore M, Milus A, et al. (2002) Wheat Stripe Rust Epidemics and Races of Puccinia striiformis f. sp. tritici in the United States in 2000. Plant Disease 86(1): 39-46. doi: 10.1094/PDIS.2002.86.1.39
Chen XM (2005) Epidemiology and control of stripe rust [Puccinia striiformis f. sp. tritici] on wheat. Canadian Journal of Plant Pathology 27: 314–337. doi: 10.1080/07060660509507230
Chen X (2013) Review Article: High-Temperature Adult-Plant Resistance, Key for Sustainable Control of Stripe Rust. American Journal of Plant Sciences 4(3): 608-627. doi: 10.4236/ajps.2013.43080
Chen W, Wellings C, Chen X, et al. (2014) Wheat stripe (yellow) rust caused by Puccinia striiformis f. sp. tritici. Molecular Plant Pathology 15: 433–446. doi: 10.1111/mpp.12116
Chen XM, Kang Z (2017) Stripe Rust. Springer Netherlands. 719 p. doi: 10.1007/978-94-024-1111-9
Chen W, Wellings C, Chen X, et al. (2014) Wheat stripe (yellow) rust caused by Puccinia striiformis f. sp. tritici. Molecular Plant Pathology 15(5): 433-446. doi: 10.1111/mpp.12116
Chen XM, Kang Z (2017a) Introduction: History of Research, Symptoms, Taxonomy of the Pathogen, Host Range, Distribution, and Impact of Stripe Rust. In: Chen, X. M. & Z. Zang (Eds.). Stripe Rust. Springer Netherlands. pp. 1-33. doi: 10.1007/978-94-024-1111-9.
Chen XM (2020) Pathogens which threaten food security: Puccinia striiformis, the wheat stripe rust pathogen. Food Security 12: 239–251. doi: 10.1007/s12571-020-01016-z
(2021) Virulence characterization of Puccinia striiformis f. sp. tritici collections from six countries in 2013 to 2020. Canadian Journal of Plant Pathology. doi: 10.1080/07060661.2021.1958259
Chen W, Zhang Z, Chen X, et al. (2021) Field Production, Germinability, and Survival of Puccinia striiformis f. sp. tritici Teliospores in China. Plant Disease 105(8): 2122-2128. doi: 10.1094/PDIS-09-20-2018-RE
Cheng P, Xu LS, Wang MN, et al. (2014) Molecular mapping of genes Yr64 and Yr65 for stripe rust resistance in hexaploid derivatives of durum wheat accessions PI 331260 and PI 480016. Theoretical and Applied Genetics 127(10): 2267–2277. doi: 10.1007/s00122-014-2378-8
Cheng J-J, Li H, Ren B, et al. (2015) Effect of canopy temperature on the stripe rust resistance of wheat. New Zealand Journal of Crop and Horticultural Science 43(4): 306-315. doi: 10.1080/01140671.2015.1098708
Cheng Y, Wu K, Yao J, et al. (2017) PSTha5a23, a candidate effector from the obligate biotrophic pathogen Puccinia striiformis f. sp. tritici, is involved in plant defense suppression and rust pathogenicity. Environmental Microbiology 19: 1717-1729. doi: 10.1111/1462-2920.13610
Cheng B, Gao X, Cao N, et al. (2022) QTL mapping for adult plant resistance to wheat stripe rust in M96-5 × Guixie 3 wheat population. J Appl Genetics 63: 265–279. doi: 10.1007/s13353-022-00686-z
Chu B, Yuan K, Wang C, et al. (2021) Effects of Wheat Cultivar Mixtures on Population Genetic Structure of Puccinia striiformis f. sp. tritici. PhytoFrontiers™ 1:4, 339-353. doi: 10.1094/PHYTOFR-01-21-0006-R
Cobo N, Pflüger L, Chen X, Dubcovsky J (2018) Mapping QTL for Resistance to New Virulent Races of Wheat Stripe Rust from Two Argentinean Wheat Cultivars. Crop Science 58: 2470-2483. doi: 10.2135/cropsci2018.04.0286
Cook NM, Chng S, Woodman TL, et al. (2021) High frequency of fungicide resistance‐associated mutations in the wheat yellow rust pathogen Puccinia striiformis f. sp. tritici. Pest Management Science 77: 3358-3371. doi: 10.1002/ps.6380
Coradini C, Piccinini F, Reimche GB, et al. (2016) Efeito de óleo essencial de laranja associados a fungicidas no controle de doenças foliares do trigo. Summa Phytopathologica 42(1): 105-106. doi: 10.1590/0100-5405/2020
Coram TE, Wang M, Chen X (2008) Transcriptome analysis of the wheat–Puccinia striiformis f. sp. tritici interaction. Molecular Plant Pathology 9: 157-169. doi: 10.1111/j.1364-3703.2007.00453.x
Corredor-Moreno P, Minter F, Davey PE, et al. (2021) The branched-chain amino acid aminotransferase TaBCAT1 modulates amino acid metabolism and positively regulates wheat rust susceptibility. The Plant Cell koab049. doi: 10.1093/plcell/koab049
Cuomo CA, Bakkeren G, Khalil HB, et al. (2016) Comparative Analysis Highlights Variable Genome Content of Wheat Rusts and Divergence of the Mating Loci. G3: Genes | Genomes | Genetics 7(2): 361-376. doi: 10.1534/g3.116.032797
Dagvadorj B, Ozketen AC, Andac A, et al. (2017) A Puccinia striiformis f. sp. tritici secreted protein activates plant immunity at the cell surface. Nature Scientific Reports 7: 1141. doi: 10.1038/s41598-017-01100-z
Dean R, Van Kan JAL, Pretorius ZA, et al. (2012). The Top 10 fungal pathogens in molecular plant pathology. Molecular Plant Pathology 13: 414–430. doi: 10.1111/j.1364-3703.2011.00783.x
deBoer GJ, Nott P, Kemmitt GM (2013) Use of Uptake Spraying Oil to improve fungicidal activity of the triazole fungicide fenbuconazole on Puccinia triticina and Puccinia striiformis rusts of wheat. Online. Plant Health Progress (online). doi: 10.1094/PHP-2013-0528-01- RS
Dehkordi HR, El Jarroudi M, Kouadio L, et al. (2020) Monitoring Wheat Leaf Rust and Stripe Rust in Winter Wheat Using High-Resolution UAV-Based Red-Green-Blue Imagery. Remote Sensing 12: 3696. doi: 10.3390/rs12223696
Devadas R, Simpfendorfer S, Backhouse D, Lamb DW (2014) Effect of stripe rust on the yield response of wheat to nitrogen. The Crop Journal 2(4): 201-206. doi: 10.1016/j.cj.2014.05.002
de Vallavieille-Pope C, Huber L, Leconte M, Goyeau H (1995) Comparative effects of temperature and interrupted wet periods on germination, penetration, and infection of Puccinia recondita f. sp. tritici and P. striiformis on wheat seedlings. Phytopathology 85: 409-15. doi: 10.1094/Phyto-85-409
de Vallavieille-Pope C, Ali S, Leconte M, et al. (2012) Virulence dynamics and regional structuring of Puccinia striiformis f. sp. tritici in France between 1984 and 2009. Plant Disease 96: 131-140. doi: 10.1094/PDIS-02-11-0078
de Vallavieille-Pope C, Bahri B, Leconte M, et al. (2018) Thermal generalist behavior of invasive Puccinia striiformis f. sp. tritici strains under current and future climate conditions. Plant Pathology (Accepted Manuscript). doi: 10.1111/ppa.12840
Ding Y, Cuddy WS, Wellings CR, et al. (2021) Incursions of divergent genotypes, evolution of virulence and host jumps shape a continental clonal population of the stripe rust pathogen Puccinia striiformis. Molecular Ecology. doi: 10.1111/mec.16182
Dobon A, Bunting DC, Cabrera-Quio LE, et al. (2016) The host-pathogen interaction between wheat and yellow rust induces temporally coordinated waves of gene expression. BMC Genomics 17: 380. doi: 10.1186/s12864-016-2684-4
Dong Z, Hegarty JM, Zhang J, et al. (2017) Validation and characterization of a QTL for adult plant resistance to stripe rust on wheat chromosome arm 6BS (Yr78). Theoretical and Applied Genetics (online): 1-11. doi: 10.1007/s00122-017-2946-9
Dracatos PM, Haghdoust R, Singh D, Park RF (2017) Genetic analysis and molecular mapping of resistance to Puccinia striiformis f. sp. pseudo-hordei in common wheat. Plant Pathology 66: 285–292. doi: 10.1111/ppa.12580
Duan X, Tellier A, Wan A, et al. (2010) Puccinia striiformis f. sp. tritici presents high diversity and recombination in the over-summering zone of Gansu, China. Mycologia 102(1): 44-53. doi: 10.3852/08-098
Eagle J, Liu Y, Naruoka Y, et al. (2022) Identification and Mapping of Quantitative Trait Loci Associated with Stripe Rust Resistance in Spring Club Wheat Cultivar JD. Plant Dis. 106(9): 2490-2497. doi: 10.1094/PDIS-12-21-2627-RE
Eddy R (2009) Logistic regression models to predict stripe rust infections on wheat and yield response to foliar fungicide application on wheat in Kansas. Master of Science, Kansas State University. Link
El Amil R, Shykoff JA, Vidal T, et al. (2022) Diversity of thermal aptitude of Middle Eastern and Mediterranean Puccinia striiformis f. sp. tritici isolates from different altitude zones. Plant Pathology 71: 1674– 1687. doi: 10.1111/ppa.13613
El Jarroudi M, Kouadio L, Bock CH, et al. (2017) A Threshold-Based Weather Model for Predicting Stripe Rust Infection in Winter Wheat. Plant Disease 101(5): 693-703. doi: 10.1094/PDIS-12-16-1766-RE
El-Sharkawy HHA, Rashad YM, Elazab NT (2023) Induction of multiple defense responses in wheat plants against stripe rust using mycorrhizal fungi and Streptomyces viridosporus HH1. BioControl 68: 525–535. doi: 10.1007/s10526-023-10207-4
Eriksson J, Henning E (1896) The cereal rusts. Nortstedt & Söner, Stockholm.
Esmail SM, Draz IS, Ashmawy MA, El-Orabey WM (2021) Emergence of new aggressive races of Puccinia striiformis f. sp. tritici causing yellow rust epiphytotic in Egypt. Physiological and Molecular Plant Pathology. doi: 10.1016/j.pmpp.2021.101612
Farber DH, Medlock J, Mundt CC (2017) Local dispersal of Puccinia striiformis f. sp. tritici from isolated source lesions. Plant Pathology 66: 28–37. doi: 10.1111/ppa.12554
Farber D (2017) The primary disease gradient of Wheat Stripe Rust (Puccinia striiformis f. sp. tritici) across spatial scales. Ph. D. Thesis, Oregon State University. 159 p.
Fetterley V, Kutcher R, Kumar S, et al. (2024), Race typing of Puccinia striiformis f. sp. tritici using an improved differential set will accelerate genetic gains for stripe rust resistance in Canada. Plant Pathol 73: 215-220. doi: 10.1111/ppa.13817
Feng JY, Wang MN, Chen XM, et al. (2015) Molecular Mapping of YrSP and Its Relationship with Other Genes for Stripe Rust Resistance in Wheat Chromosome 2BL. Phytopathology 105(9): 1206-1213. doi: 10.1094/PHYTO-03-15-0060-R
Feng J, Yao F, Wang M, et al. (2023) Molecular Mapping of Yr85 and Comparison with Other Genes for Resistance to Stripe Rust on Wheat Chromosome 1B. Plant Disease. doi: 10.1094/PDIS-11-22-2600-RE
Fetterley V, Kutcher R, Kumar S, et al. (2023) Race typing of Puccinia striiformis f. sp. tritici using an improved differential set will accelerate genetic gains for stripe rust resistance in Canada. Plant Pathol. doi: 10.1111/ppa.13817
Franco MF, Polacco AN, Campos PE, et al. (2022) Genome-wide association study for resistance in bread wheat (Triticum aestivum L.) to stripe rust (Puccinia striiformis f. sp. tritici) races in Argentina. BMC Plant Biol 22: 543. doi: 10.1186/s12870-022-03916-y
Fungicidal Control of Stripe Rust (Puccinia striiformis f.sp. tritici)
Gao P, Zhou Y, Gebrewahid TW, et al. (2024) QTL Mapping for Adult-Plant Resistance to Leaf Rust in Italian Wheat Cultivar Libellula. Plant Dis. 108(1): 13-19. doi: 10.1094/PDIS-01-23-0105-SR
Garcia LC, Machado Júnior CR, Bochnia GP, et al. (2016) Adjuvants in fungicide spraying in wheat and soybean crops. Engenharia Agrícola 36(6): 1110-1117. doi: 10.1590/1809-4430-eng.agric.v36n6p1110-1117/2016
Gaurav K, Arora S, Silva P, et al. (2021) Population genomic analysis of Aegilops tauschii identifies targets for bread wheat improvement. Nat Biotechnol. doi: 10.1038/s41587-021-01058-4
Geagea L, Huber L, Sache I (1999) Dry-dispersal and rain-splash of brown (Puccinia recondita f. sp. tritici) and yellow (P. striiformis) rust spores from infected wheat leaves exposed to simulated raindrops. Plant Pathology 48: 472–482. doi: 10.1046/j.1365-3059.1999.00372.x
Geagea L, Huber L, Sache I, et al. (2000) Influence of simulated rain on dispersal of rust spores from infected wheat seedlings. Agric. For. Meteorol. 101: 53-66. doi: 10.1016/S0168-1923(99)00155-0
Germán S, Barcellos A, Chaves M, et al. (2007) The situation of common wheat rusts in the Southern Cone of America and perspectives for control. Australian Journal of Agricultural Research 58: 620-630. doi: 10.1071/AR06149
Ghanbarnia K, Gourlie R, Amundsen E, Aboukhaddour R (2021) The changing virulence of stripe rust in Canada from 1984 to 2017. Phytopathology 2021. doi: 10.1094/PHYTO-10-20-0469-R
Gilbert B, Bettgenhaeuser J, Upadhyaya N, et al. (2018) Components of Brachypodium distachyon resistance to nonadapted wheat stripe rust pathogens are simply inherited. PLoS Genet 14(9): e1007636. doi: 10.1371/journal.pgen.1007636
Grabow (2016) Environmental conditions associated with stripe rust and leaf rust epidemics in Kansas winter wheat. PhD Thesis, Kansas State University, 130 p.
Guo ZF, Tao F, Tian W, et al. (2017) Functions of TaWRKY45 on the high-temperature resistance to stripe rust in Xiaoyan 6. J. Wheat Crops 37: 1318–1326.
Gyawali S, Verma RPS, Kumar S, et al. (2018) Seedling and adult-plant stage resistance of a world collection of barley genotypes to stripe rust. Journal of Phytopathology 166: 18–27. doi: 10.1111/jph.12655
Han DJ, Wang QL, Chen XM, et al. (2015) Emerging Yr26-Virulent Races of Puccinia striiformis f. tritici Are Threatening Wheat Production in the Sichuan Basin, China. Plant Disease 99(6): 754-760. doi: 10.1094/PDIS-08-14-0865-RE
Holden S, Bergum M, Green P, et al. (2022) A lineage-specific Exo70 is required for receptor kinase–mediated immunity in barley. Science Advances 8(27): eabn7258. doi: 10.1126/sciadv.abn7258
Hovmøller MS (2007) Sources of seedling and adult plant resistance to Puccinia striiformis f.sp. tritici in European wheats. Plant Breeding 126: 225-233. doi: 10.1111/j.1439-0523.2007.01369.x
Hovmøller MS, Yahyaoui AH, Milus EA, Justesen AF (2008) Rapid global spread of two aggressive strains of a wheat rust fungus. Molecular Ecology 17, 3818-3826. doi: 10.1111/j.1365-294X.2008.03886.x
Hovmøller MS, Walter S, Justesen AF (2010) Escalating threat of wheat rusts. Science 329 (5990): 369. doi: 10.1126/science.1194925
Hovmøller MS, Sørensen CK, Walter S, Justesen AF (2011) Diversity of Puccinia striiformis on cereals and grasses. Annual Review of Phytopathology 49: 197–217. doi: 10.1146/annurev-phyto-072910-095230
Hovmøller MS, Walter S, Bayles RA, et al. (2016) Replacement of the European wheat yellow rust population by new races from the centre of diversity in the near-Himalayan region. Plant Pathology 65: 402–411. doi: 10.1111/ppa.12433
Hovmøller MS, Rodriguez-Algaba J, Thach T, et al. (2017) Report for Puccinia striiformis race analyses and molecular genotyping 2016, Global Rust Reference Center (GRRC), Aarhus University, Flakkebjerg, DK- 4200 Slagelse, Denmark. 2 February, 2017.
Hovmøller et al. (2017) Race Typing of Puccinia striiformis on Wheat. In: Wheat Rust Diseases: Methods and Protocols, ed. S. Periyannan. Springer, p. 29-40. doi: 10.1007/978-1-4939-7249-4
Hovmøller MS, Thach T, Justesen AF (2023) Global dispersal and diversity of rust fungi in the context of plant health. Current Opinion in Microbiology 71: 102243. doi: 10.1016/j.mib.2022.102243
Hu Y, Tao F, Su C, et al. (2020) NBS-LRR gene TaRPS2 is positively associated with the high-temperature seedling plant resistance of wheat against Puccinia striiformis f. sp. tritici. Phytopathology. doi: 10.1094/PHYTO-03-20-0063-R
Hu C, Wang F, Feng J, et al. (2022) Identification and molecular mapping of YrBm for adult plan resistance to stripe rust in Chinese wheat landrace Baimangmai. Theor Appl Genet 135: 2655–2664. doi: 10.1007/s00122-022-04139-3
Hu Y, Su C, Zhang Y, et al. (2022), A Puccinia striiformis f. sp. tritici effector inhibits high-temperature seedling-plant resistance in wheat. Plant J 112: 249-267. doi: 10.1111/tpj.15945
Hu Y, Zhang Y, Lu K, et al. (2023) Identification of high-temperature resistance to stripe rust and molecular detection of Yr genes in Chinese core collections of common wheat. Crop Protection 164: 106136. doi: 10.1016/j.cropro.2022.106136
Hu X, Lin J, Li Y, et al. (2024) Dynamics of Puccinia striiformis f. sp. tritici Urediniospores and Its Relationship with Meteorological Factors in Mianyang, China. Phytopathology. doi: 10.1094/PHYTO-01-24-0012-R
Huang S, Zuo S, Zheng D, et al. (2019) Three formae speciales of Puccinia striiformis were identified as heteroecious rusts based on completion of sexual cycle on Berberis spp. under artificial inoculation. Phytopathology Research 14. doi: 10.1186/s42483-019-0021-y
Huang S, Zhang Y, Ren H, et al. (2022) High density mapping of wheat stripe rust resistance gene QYrXN3517-1BL using QTL mapping, BSE-seq and candidate gene analysis, PREPRINT. doi: 10.21203/rs.3.rs-1969279/v1
, , , et al (2023) TaUAM3, a UDP-Ara mutases protein, positively regulates wheat resistance to the stripe rust fungus. Food and Energy Security 12: e456. doi: 10.1002/fes3.456
Hubbard A, Lewis CM, Yoshida K, et al. (2015) Field pathogenomics reveals the emergence of a diverse wheat yellow rust population. Genome Biology 16: 23. doi: 10.1186/s13059-015-0590-8
Huerta-Espino J, Singh RP (2017) First Detection of Virulence in Puccinia striiformis f. sp. tritici to Wheat Resistance Genes Yr10 and Yr24 (=Yr26) in Mexico. Plant Disease 101(9): 1676-1676. doi: 10.1094/PDIS-04-17-0532-PDN
Iqbal M, Semagn K, Jarquin D, et al. (2022) Identification of Disease Resistance Parents and Genome-Wide Association Mapping of Resistance in Spring Wheat. Plants 11(21): 2905. doi: 10.3390/plants11212905
International Wheat Genome Sequencing Consortium (IWGSC); IWGSC RefSeq principal investigators:, Appels R, et al. (2018) Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science 361(6403): eaar7191. doi: 10.1126/science.aar7191
Ji F, Zhang J, Chen X, et al. (2023) Effects of Flubeneteram on Inhibiting the Development of Puccinia striiformis f. sp. tritici in Wheat Leaves. J Agric Food Chem. doi: 10.1021/acs.jafc.3c00499
Jiang B, Wang C, Guo C, et al. (2022) Genetic Relationships of Puccinia striiformis f. sp. tritici in Southwestern and Northwestern China. Microbiol Spectr.: e0153022. doi: 10.1128/spectrum.01530-22
Jiang P, Wu L, Ren Y, et al. (2023) Identification of adult plant yellow rust resistance QTLs in Jiangsu wheat varieties Ningmai 9 and Yangmai 158. Plant Pathology 00: 1– 8. doi: 10.1111/ppa.13709
Jiao M, Tan C, Wang L, et al. (2017) Basidiospores of Puccinia striiformis f. sp. tritici succeed to infect barberry, while Urediniospores are blocked by non-host resistance. Protoplasma (online): 1-10. doi: 10.1007/s00709-017-1114-z
Jin Y, Szabo LJ, Carson M (2010) Century-old mystery of Puccinia striiformis life history solved with the identification of Berberis as an Alternate Host. Phytopathology 100: 432-435. doi: 10.1094/PHYTO-100-5-0432
Jin Y (2011) Role of Berberis spp. as alternate hosts in generating new races of Puccinia graminis and P. striiformis. Euphytica 179(1): 105–108. doi: 10.1007/s10681-010-0328-3
Juliana P, Singh RP, Singh PK, et al. (2018) Genome-wide association mapping for resistance to leaf rust, stripe rust and tan spot in wheat reveals potential candidate genes. Theor Appl Genet. 1-18. doi: 10.1007/s00122-018-3086-6
Kanwal M, Qureshi N, Gessese M, et al. (2021) An adult plant stripe rust resistance gene maps on chromosome 7A of Australian wheat cultivar Axe. Theor Appl Genet 134: 2213–2220. doi: 10.1007/s00122-021-03818-x
Kankwatsa P, Singh D, Thomson PC, et al. (2017) Characterization and genome-wide association mapping of resistance to leaf rust, stem rust and stripe rust in a geographically diverse collection of spring wheat landraces. Molecular Breeding 37: 113. doi: 10.1007/s11032-017-0707-8
King JE (1976) Relationship between Yield Loss and Severity of Yellow Rust Recorded on a Large Number of Single Stems of Winter Wheat. Plant Pathology 25: 172–177. doi: 10.1111/j.1365-3059.1976.tb01953.x
Klymiuk V, Fatiukha A, Fahima T (2019) Wheat tandem kinases provide insights on disease‐resistance gene flow and host–parasite co‐evolution. The Plant Journal 98: 667-679. doi: 10.1111/tpj.14264
Klymiuk V, Fatiukha A, Raats D, et al. (2020) Three previously characterized resistances to yellow rust are encoded by a single locus Wtk1. Journal of Experimental Botany 71(9): 2561-2572. doi: 10.1093/jxb/eraa020
Knorr K, Jørgensen LN, Nicolaisen M (2019) Fungicides have complex effects on the wheat phyllosphere mycobiome. PLoS ONE 14(3): e0213176. doi: 10.1371/journal.pone.0213176
Kolmer J, Chen X, Jin Y (2009) Diseases which Challenge Global Wheat Production-The Wheat Rusts, in Wheat Science and Trade (ed. Carver BF), Wiley-Blackwell, Oxford, UK. doi: 10.1002/9780813818832.ch5
Kuzdraliński A, Kot A, Szczerba H, et al. (2017) Novel PCR Assays for the Detection of Biological Agents Responsible for Wheat Rust Diseases: Puccinia triticina and Puccinia striiformis f. sp. tritici. Journal of Molecular Microbiology and Biotechnology 27:299-305. doi: 10.1159/000481799
Landis DA, Saidov N, Jaliov A, et al. (2016) Demonstration of an Integrated Pest Management Program for Wheat in Tajikistan. Journal of Integrated Pest Management 7(1): 11. doi: 10.1093/jipm/pmw010
Lannou C, Hubert P, Gimeno C (2005) Competition and interactions among stripe rust pathotypes in wheat-cultivar mixtures. Plant Pathology 54: 699–712. doi: 10.1111/j.1365-3059.2005.01251.x
Lei Y, Yao Z, He D (2018) Automatic detection and counting of urediniospores of Puccinia striiformis f. sp. tritici using spore traps and image processing. Scientific Reports 8: 13647. doi: 10.1038/s41598-018-31899-0
Li M, Zhang Y, Chen W. et al. (2021) Evidence for Yunnan as the major origin center of the dominant wheat fungal pathogen Puccinia striiformis f. sp. tritici. Australasian Plant Pathol. doi: 10.1007/s13313-020-00770-0
Li Y, Liu L, Wang M, et al. (2022) Characterization and Molecular Mapping of a Gene Conferring High-temperature Adult-plant Resistance to Stripe Rust Originally from Aegilops ventricosa. Plant Disease. doi: 10.1094/PDIS-06-22-1419-RE
Li Y, Dai J, Zhang T, et al. (2023) Genomic analysis, trajectory tracking and field investigation of wheat stripe rust pathogen reveal sources and their migration routes contributing to disease epidemics in China. Plant Communications 100563. doi: 10.1016/j.xplc.2023.100563
Li Y, Dai J, Zhang T, et al. (2023) Genomic analysis, trajectory tracking, and field surveys reveal sources and long-distance dispersal routes of wheat stripe rust pathogen in China. Plant Communications: 100563. doi: 10.1016/j.xplc.2023.100563
, , , et al. (2023) TaRBP1 stabilizes TaGLTP and negatively regulates stripe rust resistance in wheat. Molecular Plant Pathology 24: 1205–1219. doi: 10.1111/mpp.13364
Li Y, Wang M, Hu X, Chen X (2023) Identification of a Locus for High-Temperature Adult-Plant Resistance to Stripe Rust in the Wheat Yr8 Near-Isogenic Line through Mutagenesis and Molecular Mapping. Plant Disease. doi: 10.1094/PDIS-10-23-2037-RE
Li Y, Yang J, Zhang J, et al. (2025) Identification of QTLs for adult-plant stripe rust resistance in Chinese wheat landrace Yizhanghongkemai and assessment of their utility for decreasing yield loss. PREPRINT (Version 1). doi: 10.21203/rs.3.rs-5643654/v1
Lin X, N’Diaye A, Walkowiak S, et al. (2018) Genetic analysis of resistance to stripe rust in durum wheat (Triticum turgidum L. var. durum). PLOS ONE. 13(9): e0203283. doi: 10.1371/journal.pone.0203283
Lintz J, Dubrulle G, Cawston E, et al. (2022) A Short Review of Anti-Rust Fungi Peptides: Diversity and Bioassays. Front. Agron. 4: 966211. doi: 10.3389/fagro.2022.966211
Lindquist JC (1982) Royas de la República Argentina y zonas limítrofes. Colección científica del INTA. 574 p.
Liu M, Hambleton S (2010) Taxonomic study of stripe rust, Puccinia striiformis sensu lato, based on molecular and morphological evidence. Fungal Biology 114(10): 881-899. doi: 10.1016/j.funbio.2010.08.005
Liu M, Peng Y, Li H, et al. (2016) TaSYP71, a Qc-SNARE, Contributes to Wheat Resistance against Puccinia striiformis f. sp. tritici. Frontiers in Plant Science 7: 544. doi: 10.3389/fpls.2016.00544
Liu T, Wan A, Liu D, Chen X (2017) Changes of Races and Virulence Genes in Puccinia striiformis f. sp. tritici, the Wheat Stripe Rust Pathogen, in the United States from 1968 to 2009. Plant Disease 101(8): 1522-1532. doi: 10.1094/PDIS-12-16-1786-RE
Liu W, Maccaferri M, Chen X, et al. (2017) Genome-wide association mapping reveals a rich genetic architecture of stripe rust resistance loci in emmer wheat (Triticum turgidum ssp. dicoccum). Theoretical and Applied Genetics (online): 1-22. doi: 10.1007/s00122-017-2957-6
Liu W, Maccaferri M, Rynearson S, et al. (2017) Novel Sources of Stripe Rust Resistance Identified by Genome-Wide Association Mapping in Ethiopian Durum Wheat (Triticum turgidum ssp. durum). Front. Plant Sci. 8:774. doi: 10.3389/fpls.2017.00774
Liu L, Wang M, Zhang Z, et al. (2020) Identification of Stripe Rust Resistance Loci in U.S. Spring Wheat Cultivars and Breeding Lines Using Genome-Wide Association Mapping and Yr Gene Markers. Plant Disease 104(8): 2181-2192. doi: 10.1094/PDIS-11-19-2402-RE
Liu T, Bai Q, Wang M, et al. (2021) Genotyping Puccinia striiformis f. sp. tritici Isolates with SSR and SP-SNP Markers Reveals Dynamics of the Wheat Stripe Rust Pathogen in the United States from 1968 to 2009 and Identifies Avirulence Associated Markers. Phytopathology. doi: 10.1094/PHYTO-01-21-0010-R
Liu P, Myo T, Ma W, et al. (2016) TaTypA, a Ribosome-Binding GTPase Protein, Positively Regulates Wheat Resistance to the Stripe Rust Fungus. Front. Plant Sci. 7: 873. doi: 10.3389/fpls.2016.00873
Livbjerg M (2025) A Global Threat: Epidemiology and Management of Yellow Rust on Wheat. CABI Plant Health Cases. doi: 10.1079/planthealthcases.2025.0011
Long XY, Liu YX, Rocheleau H, et al. (2011) Identification and Validation of Internal Control Genes for Gene Expression in Wheat Leaves Infected by Strip Rust. International Journal of Plant Breeding and Genetics 5: 255-267. doi: 10.3923/ijpbg.2011.255.267
Lüttringhaus S, Zetzsche H, Wittkop B, et al. (2021) Resistance Breeding Increases Winter Wheat Gross Margins–An Economic Assessment for Germany. Front. Agron. 3: 730894. doi: 10.3389/fagro.2021.730894
Lyon B, Broders K (2017) Impact of climate change and race evolution on the epidemiology and ecology of stripe rust in central and eastern U.S. and Canada. Canadian Journal of Plant Pathology. doi: 10.1080/07060661.2017.13687+
Ma J, Huang X, Wang X, et al. (2009) Identification of expressed genes during compatible interaction between stripe rust (Puccinia striiformis) and wheat using a cDNA library. BMC Genomics 10: 586. doi: 10.1186/1471-2164-10-586
Ma M, Jin Y, Chen X, et al. (2025) Virulence Characterization and Population Structure of Puccinia striiformis f. sp. tritici in Henan Province, China. Plant Dis. doi: 10.1094/PDIS-11-24-2352-RE
Marchal C, Zhang J, Zhang P, et al. (2018) BED-domain containing immune receptors confer diverse resistance spectra to yellow rust. Nature Plants 4: 662–668 . doi: 10.1038/s41477-018-0236-4
Markell SG, Milus EA (2008) Emergence of a Novel Population of Puccinia striiformis f. sp. tritici in Eastern United States. Phytopathology 98(6): 632-639. doi: 10.1094/PHYTO-98-6-0632
Mashabela MD, Tugizimana F, Steenkamp PA, et al. (2023) Metabolomic evaluation of PGPR defence priming in wheat (Triticum aestivum L.) cultivars infected with Puccinia striiformis f. sp. tritici (stripe rust). Front. Plant Sci. 14: 1103413. doi: 10.3389/fpls.2023.1103413
Mashabela MD, Tugizimana F, Steenkamp PA, et al. (2023) Metabolite profiling of susceptible and resistant wheat (Triticum aestivum) cultivars responding to Puccinia striiformis f. sp. tritici infection. BMC Plant Biol 23: 293. doi: 10.1186/s12870-023-04313-9
Mboup M, Bahri B, Leconte M, et al. (2012) Genetic structure and local adaptation of European wheat yellow rust populations: the role of temperature-specific adaptation. Evol Appl. 5(4): 341-52. doi: 10.1111/j.1752-4571.2011.00228.x
McLean M, Henry F, Hollaway G (2010) Stripe rust management in wheat. BCG 2010 Season Research Results: 140-142.
Mehmood S, Sajid M, Zhao J, et al. (2018) Identification of Berberis species Collected from the Himalayan Region of Pakistan Susceptible to Puccinia striiformis f. sp. tritici. Plant Disease (accepted). doi: 10.1094/PDIS-01-18-0154-RE
Z (2020) Alternate hosts and their impact on genetic diversity of Puccinia striiformis f. sp. tritici and disease epidemics, Journal of Plant Interactions 15: 1, 153-165. doi: 10.1080/17429145.2020.1771445
Mehmood S, Sajid M, Zhao J, et al. (2020) Alternate hosts of Puccinia striiformis f. sp. tritici and their role. Pathogens 9(6): 434. doi: 10.3390/pathogens9060434
(2020) Management of yellow rust (Puccinia striiformis f. sp. tritici) and stem rust (Puccinia graminis f. sp tritici) of bread wheat through host resistance and fungicide application in Southern Ethiopia. Cogent Food & Agriculture, 6: 1. doi: 10.1080/23311932.2020.1739493
Miedaner T, Schmid JE, Flath K, et al. (2017) A multiple disease test for field-based phenotyping of resistances to Fusarium head blight, yellow rust and stem rust in wheat. European Journal of Plant Pathology : 1-11. doi: 10.1007/s10658-017-1386-3
Millet E, Manisterski J, Ben-Yehuda P, et al. (2014) Introgression of leaf rust and stripe rust resistance from Sharon goatgrass (Aegilops sharonensis Eig) into bread wheat (Triticum aestivum L.). Genome 57: 309-316. doi: 10.1139/gen-2014-0004
Milus EA, Seyran E, McNew R (2006) Aggressiveness of Puccinia striiformis f. sp. tritici isolates in the south-central United States. Plant Disease 90: 847-852. doi: 10.1094/PD-90-0847
Milus EA, Kristensen K, Hovmøller MS (2009) Evidence for increased aggressiveness in a recent widespread strain of Puccinia striiformis f. sp. tritici causing stripe rust of wheat. Phytopathology 99: 89-94. doi: 10.1094/PHYTO-99-1-0089
Mojerlou S, Moeller M, Schwessinger B, Rodriguez-Algaba J (2024) Beyond Asexual: Genomics-Driven Progress in Unveiling Sexual Reproduction in Cereal Rust Fungi. Mol Plant Microbe Interact. doi: 10.1094/MPMI-10-24-0122-FI
Moscou MJ, Van Esse PH (2017) The quest for durable resistance. Science 358(6370): 1541-1542. doi: 10.1126/science.aar4797
Mourad AMI, Abou-Zeid MA, Eltaher S, et al. (2021) Identification of Candidate Genes and Genomic Regions Associated with Adult Plant Resistance to Stripe Rust in Spring Wheat. Agronomy 11(12): 2585. doi: 10.3390/agronomy11122585
Mueller BD, Groves CL, Conley SP, et al. (2020) Integrated Management of Stripe Rust and Overwintering of Puccinia striiformis f. sp. tritici in Wisconsin. Plant Health Progress 21: 205-211. doi: 10.1094/PHP-04-20-0027-RS
Mueth NA, Ramachandran SR, Hulbert SH (2015) Small RNAs from the wheat stripe rust fungus (Puccinia striiformis f.sp. tritici). BMC Genomics 16(1): 718. doi: 10.1186/s12864-015-1895-4
Mukhtar S, Khan MA, Paddar BA, et al. (2015) Molecular characterization of wheat germplasm for stripe rust resistance genes (Yr5, Yr10, Yr15 & Yr18) and identification of candidate lines for stripe rust breeding in Kashmir. Indian Journal of Biotechnology 14: 241-248.
Muleta KT, Rouse MN, Rynearson S, et al. (2017) Characterization of molecular diversity and genome-wide mapping of loci associated with resistance to stripe rust and stem rust in Ethiopian bread wheat accessions. BMC Plant Biology 17:134. doi: 10.1186/s12870-017-1082-7
Muleta KT, Bulli P, Rynearson S, et al. (2017a) Loci associated with resistance to stripe rust (Puccinia striiformis f. sp. tritici) in a core collection of spring wheat (Triticum aestivum). PLoS ONE 12(6): e0179087. doi: 10.1371/journal.pone.0179087
Murray G, Wellings C, Simpfendorfer S, Cole C (2005) Stripe rust: Understanding the disease in wheat. Department of Primari Industries, State of New South Wales, Australia.
Naruoka Y, Ando K, Bulli P, et al. (2016) Identification and Validation of SNP Markers Linked to the Stripe Rust Resistance Gene Yr5 in Wheat. Crop Science 56(6): 3055-3065. doi: 10.2135/cropsci2016.03.0189
Norman M, Bariana H, Bansal U, Periyannan S (2023) The Keys to Controlling Wheat Rusts: Identification and Deployment of Genetic Resistance. Phytopathology 113(4): 667-677. doi: 10.1094/PHYTO-02-23-0041-IA
Nsabiyera V, Bariana HS, Qureshi N, et al. (2018) Characterisation and mapping of adult plant stripe rust resistance in wheat accession Aus27284. Theor Appl Genet 1-9. doi: 10.1007/s00122-018-3090-x
Nyamesorto B, Zhang H, Rouse M, et al. (2022) A transcriptomic-guided strategy used in identification of a wheat rust pathogen target and modification of the target enhanced host resistance to rust pathogens. Front. Plant Sci. 13: 962973. doi: 10.3389/fpls.2022.962973
Omara RI, Mazrou YSA, Elsayed A, et al. (2022) MISSR: A Mentoring Interactive System for Stripe Rust. Agronomy 12(10): 2416. doi: 10.3390/agronomy12102416
Peng F, Si M, Zizhu Y, et al. (2020) Rapid Quantification of Fungicide Effectiveness on Inhibiting Wheat Stripe Rust Pathogen (Puccinia striiformis f. sp. tritici). Plant Disease 104(9): 2434-2439. doi: 10.1094/PDIS-09-19-1836-RE
Periyannan S (2017) Wheat Rust Diseases. Methods and Protocols. Methods in Molecular Biology Volume 1659 2017. Springer New York. 291 p. doi: 10.1007/978-1-4939-7249-4
Perronne R, Dubs F, de Vallavieille-Pope C, et al. (2021) Spatiotemporal Changes in Varietal Resistance to Wheat Yellow Rust in France Reveal an Increase in Field Resistance Level During the Period 1985-2018. Phytopathology:PHYTO05200187R. doi: 10.1094/PHYTO-05-20-0187-R
Prahl KC, Klink H, Hasler M, et al. (2022) Can Decision Support Systems Help Improve the Sustainable Use of Fungicides in Wheat? Sustainability 14(23): 15599. doi: 10.3390/su142315599
Pretorius ZA, Lan CX, Prins R, et al. (2017) Application of remote sensing to identify adult plant resistance loci to stripe rust in two bread wheat mapping populations. Precision Agriculture 18(4): 411-428. doi: 10.1007/s11119-016-9461-x
Qi T, Zhu X, Tan C, et al. (2017) Host-induced gene silencing of an important pathogenicity factor PsCPK1 in Puccinia striiformis f. sp. tritici enhances resistance of wheat to stripe rust. Plant Biotechnol J. Accepted Author Manuscript. doi:10.1111/pbi.12829
Qi T, Guo J, Liu P, et al. (2019) Stripe Rust Effector PstGSRE1 Disrupts Nuclear Localization of ROS-Promoting Transcription Factor TaLOL2 to Defeat ROS-Induced Defense in Wheat. Molecular Plant 12: 1624-1638. doi: 10.1016/j.molp.2019.09.010
Qureshi N, Bariana H, Forrest K, et al. (2017) Fine mapping of the chromosome 5B region carrying closely linked rust resistance genes Yr47 and Lr52 in wheat. Theoretical and Applied Genetics 130(3): 495-504. doi: 10.1007/s00122-016-2829-5
Rahmatov M (2016) Genetic Characterisation of Novel Resistance Alleles to Stem Rust and Stripe Rust in Wheat-Alien Introgression Lines. Alnarp: Swedish University of Agricultural Sciences. LINK
Rahmatov M, Hovmøller MS, Nazari K, et al. (2017) Seedling and adult plant stripe rust resistance in diverse wheat–alien introgression lines. Crop Science 57(4): 2032-2042. doi: 10.2135/cropsci2016.08.0664
Rahmatov M, Otambekova M, Muminjanov H, et al. (2019) Characterization of stem, stripe and leaf rust resistance in Tajik bread wheat accessions. Euphytica 215: 55. doi: 10.1007/s10681-019-2377-6
Riella V, Rodriguez-Algaba J, García R, et al. (2024) New Races with Wider Virulence Indicate Rapid Evolution of Puccinia striiformis f. sp. tritici in the Southern Cone of America. Plant Dis. doi: 10.1094/PDIS-02-24-0320-RE
Rodríguez-García FM, Huerta-Espino J, Villaseñor-Mir HE, et al. (2010) Análisis de virulencia de la roya amarilla (Puccinia striiformis f. sp. tritici) del trigo (Triticum aestivum L.) en los valles altos de México. Agrociencia 44 (4). LINK
Rodriguez-Algaba J, Walter S, Sørensen CK, et al. (2014) Sexual structures and recombination of the wheat rust fungus Puccinia striiformis on Berberis vulgaris. Fungal Genetics and Biology 70: 77-85. doi: 10.1016/j.fgb.2014.07.005
Rodriguez-Algaba J, Sørensen CK, Labouriau R, et al. (2017) Genetic diversity within and among aecia of the wheat rust fungus Puccinia striiformis on the alternate host Berberis vulgaris. Fungal Biology 121(6–7): 541-549. doi: 10.1016/j.funbio.2017.03.003
Rodriguez-Algaba J, Hovmøller MS, Villegas D, et al. (2021) Two indigenous Berberis species from Spain were confirmed as alternate hosts of the yellow rust fungus Puccinia striiformis f. sp. tritici. Plant Disease. doi: 10.1094/PDIS-02-21-0269-SC
Roelfs AP (1985) Wheat and rye stem rust. pp. 3–37. In: Roelfs, A. P., Bushnell, W. R. (eds.), The Cereal Rusts Vol. II: Diseases, Distribution, Epidemiology, and Control. Orlando, FL: Academic.
Roelfs AP, Singh RP, Saari EE (1992) Rust Diseases of Wheat: Concepts and methods of disease management. Mexico, D.F.: CIMMYT. 81 pages.
Rosewarne GM, Herrera-Foessel SA, Singh RP, et al. (2013) Quantitative trait loci of stripe rust resistance in wheat. Theoretical and Applied Genetics 126(10): 2427–2449. doi: 10.1007/s00122-013-2159-9
Różewicz M, Wyzińska M, Grabiński J (2021) The Most Important Fungal Diseases of Cereals—Problems and Possible Solutions. Agronomy 11(4): 714. doi: 10.3390/agronomy11040714
Ruan C, Dong Y, Huang W, et al. (2022) Integrating Remote Sensing and Meteorological Data to Predict Wheat Stripe Rust. Remote Sensing 14(5): 1221. doi: 10.3390/rs14051221
(2000) Short-distance dispersal of wheat rust spores. Agronomie 20: 757-767. doi: 10.1051/agro:2000102
Saeed M, Ullah F, Shah L, et al. (2022) Identification of Three Novel QTLs Associated with Yellow Rust Resistance in Wheat (Triticum aestivum L.) Anong-179/Khaista-17 F2 Population. Sustainability 14(12): 7454. doi: 10.3390/su14127454
Sapoukhina N, Paillard S, Dedryver F, de Vallavieille-Pope C (2013) Quantitative plant resistance in cultivar mixtures: wheat yellow rust as a modeling case study. New Phytologist 200: 888–897. doi: 10.1111/nph.12413
Savadi S, Prasad P, Kashyap PL, Bhardwaj SC (2017) Molecular breeding technologies and strategies for rust resistance in wheat (Triticum aestivum) for sustained food security. Plant Pathology (Accepted). doi: 10.1111/ppa.12802
Schwessinger B (2016) Fundamental wheat stripe rust research in the 21st century. New Phytologist (online). doi: 10.1111/nph.14159
Schwessinger B, Sperschneider J, Cuddy WS, et al. (2018) A near-complete haplotype-phased genome of the dikaryotic wheat stripe rust fungus Puccinia striiformis f. sp. tritici reveals high interhaplotype diversity. mBio 9: e02275-17. doi: 10.1128/mBio.02275-17
Schwessinger B, Chen YJ, Tien R, et al. (2020) Distinct Life Histories Impact Dikaryotic Genome Evolution in the Rust Fungus Puccinia striiformis Causing Stripe Rust in Wheat. Genome Biology and Evolution 12: 597–617. doi: 10.1093/gbe/evaa071
Semagn K, Crossa J, Cuevas J, et al. (2022) Comparison of single-trait and multi-trait genomic predictions on agronomic and disease resistance traits in spring wheat. Theor Appl Genet. doi: 10.1007/s00122-022-04147-3
Semagn K, Iqbal M, Jarquin D, et al. (2022) Genomic Prediction Accuracy of Stripe Rust in Six Spring Wheat Populations by Modeling Genotype by Environment Interaction. Plants 11(13): 1736. doi: 10.3390/plants11131736
Semagn K, Iqbal M, Jarquin D, et al. (2022) Genomic Predictions for Common Bunt, FHB, Stripe Rust, Leaf Rust, and Leaf Spotting Resistance in Spring Wheat. Genes 13(4): 565. doi: 10.3390/genes13040565
Severns PM (2022) Dispersal Kernel Type Highly Influences Projected Relationships for Plant Disease Epidemic Severity When Outbreak and At-Risk Populations Differ in Susceptibility. Life 12(11): 1727. doi: 10.3390/life12111727
Sharma-Poudyal D, Chen XM (2011) Models for Predicting Potential Yield Loss of Wheat Caused by Stripe Rust in the U.S. Pacific Northwest. Phytopathology 101(5): 544-554. doi: 10.1094/PHYTO-08-10-0215
Sharma-Poudyal D, Chen XM, Wan AM, et al. (2013) Virulence characterization of international collections of the wheat stripe rust pathogen, Puccinia striiformis f. sp. tritici. Plant Disease 97: 379-386. doi: 10.1094/PDIS-01-12-0078-RE
Sharma RC, Nazari K, Amanov A, et al. (2016) Reduction of Winter Wheat Yield Losses Caused by Stripe Rust through Fungicide Management. Journal of Phytopathology 164: 671–677. doi: 10.1111/jph.12490
Shi Y, Bao X, Song X, et al. (2023) The Leucine-Rich Repeat Receptor-Like Kinase Protein TaSERK1 Positively Regulates High-Temperature Seedling Plant Resistance to Puccinia striiformis f. sp. tritici by Interacting with TaDJA7. Phytopathology 113(7): 1325-1334. doi: 10.1094/PHYTO-11-22-0429-R
Singh R, Mahmoudpour A, Rajkumar M, Narayana R (2017) A review on stripe rust of wheat, its spread, identification and management at field level. Research on Crops 18(3): 528-533. doi: 10.5958/2348-7542.2017.00091.2
Singh J, Chhabra B, Raza A, et al. (2023) Important wheat diseases in the US and their management in the 21st century. Front. Plant Sci. 13:1010191. doi: 10.3389/fpls.2022.1010191
Sisson AJ, Allen T, Andersen K, et al. (2025) Estimated yield reductions and economic losses on wheat caused by disease from 2018 through 2021. Plant Health Progress. doi: 10.1094/PHP-09-24-0087-RS
Siyoum GZ, Zeng Q, Zhao J, et al. (2019) Inheritance of Virulence and Linkages of Virulence Genes in an Ethiopian Isolate of the Wheat Stripe Rust Pathogen (Puccinia striiformis f. sp. tritici) Determined Through Sexual Recombination on Berberis holstii. Plant Disease 103(9): 2451-2459. doi: 10.1094/PDIS-02-19-0269-RE
Song J, Carver BF, Powers C, et al. (2017) Practical application of genomic selection in a doubled-haploid winter wheat breeding program. Mol Breeding (2017) 37: 117. doi: 10.1007/s11032-017-0715-8
Sørensen CK, Labouriau R, Hovmøller MS (2017) Temporal and Spatial Variability of Fungal Structures and Host Responses in an Incompatible Rust–Wheat Interaction. Frontiers in Plant Science 8: 484. doi: 10.3389/fpls.2017.00484
Souza BJRd, Perez PH, Bauer FC, et al. (2014) Adjuvantes em pulverizações de fungicidas na cultura do trigo. Ciência Rural 44(8): 1398-1403. doi: 10.1590/0103-8478cr20131099
Steele KA, Humphreys E, Wellings CR, Dickinson MJ (2001) Support for a stepwise mutation model for pathogen evolution in Australasian Puccinia striiformis f.sp. tritici by use of molecular markers. Plant Pathology 50: 174-180. doi: 10.1046/j.1365-3059.2001.00558.x
Sucher J, Menardo F, Praz CR, et al. (2017) Transcriptional profiling reveals no response of fungal pathogens to the durable, quantitative Lr34 disease resistance gene of wheat. Plant Pathology (in press). doi: 10.1111/ppa.12797
Suffert F (2024) Disruptive effect of rainfalls on the diurnal periodicity of airborne wheat rust spore under field conditions. bioRxiv 2024.10.03.616546; doi: 10.1101/2024.10.03.616546
Sun C, Liu YK, Chao KX, et al. (2018) Characterization and molecular mapping of stripe rust resistance in wheat – Psathyrostachys huashanica introgression line H9015-17-1-9-6. Canadian Journal of Plant Pathology. doi: 10.1080/07060661.2018.1523230
Tang Z, Wang M, Schirrmann M, et al. (2023) Affordable High Throughput Field Detection of Wheat Stripe Rust Using Deep Learning with Semi-Automated Image Labeling. Computers and Electronics in Agriculture 207: 107709. doi: 10.1016/j.compag.2023.107709
, , , et al. (2020) Genome‐wide association study of resistance to PstS2 and warrior races of Puccinia striiformis f. sp. tritici (stripe rust) in bread wheat landraces. Plant Genome. e20066. doi: 10.1002/tpg2.20066
Thach T, Ali S, de Vallavieille-Pope C, et al. (2016) Worldwide population structure of the wheat rust fungus Puccinia striiformis in the past. Fungal Genetics and Biology 87: 1-8. doi: 10.1016/j.fgb.2015.12.014
Tischler YK, Thiessen E, Hartung E (2018) Early optical detection of infection with brown rust in winter wheat by chlorophyll fluorescence excitation spectra. Computers and Electronics in Agriculture 146: 77–85. doi: 10.1016/j.compag.2018.01.026
TRIGO. Actualización técnica 2017. INTA ediciones. 200 p.
Ullah K, Khan NU, Gul R, et al. (2016) Genetic effects for controlling stripe rust (Puccinia striiformis f. sp. tritici) resistance in wheat through joint segregation analysis. Acta Scientiarum. Agronomy 38(3): 317-328. doi: 10.4025/actasciagron.v38i3.28347
Vergara-Diaza O, Kefauvera SC, Elazaba A, et al. (2015) Grain yield losses in yellow-rusted durum wheat estimated using digital and conventional parameters under field conditions. The Crop Journal 3 200-210. doi: 10.1016/j.cj.2015.03.003
(2022) Success and failure of invasive races of plant pathogens: The case of Puccinia striiformis f. sp. tritici in France. Plant Pathology 00, 1– 12. doi: 10.1111/ppa.13581
Villaréal LMMA, Lannou C, Vallavieille-Pope C, Neema C (2002) Genetic Variability in Puccinia striiformis f. sp. tritici Populations Sampled on a Local Scale during Natural Epidemics. Applied Environmental Microbiology 68(12): 6138-6145. doi: 10.1128/AEM.68.12.6138-6145.2002
Vikram P, Sehgal D, Sharma A, et al. (2021) Genome-wide association analysis of Mexican bread wheat landraces for resistance to yellow and stem rust. PLoS ONE 16(1): e0246015. doi: 10.1371/journal.pone.0246015
Vo Van-Zivkovic N, Dinglasan E, Tong J, et al. (2025) A large-scale multi-environment study dissecting adult-plant resistance haplotypes for stripe rust resistance in Australian wheat breeding populations. Theor Appl Genet 138: 72. doi: 10.1007/s00122-025-04859-2
Walter S, Ali S, Kemen E, et al. (2016) Molecular markers for tracking the origin and worldwide distribution of invasive strains of Puccinia striiformis. Ecology and Evolution 6(9): 2790-2804. doi: 10.1002/ece3.2069
Wan Q, Liang J, Luo Y, Ma Z (2015) Population Genetic Structure of Puccinia striiformis in Northwestern China. Plant Dis. 99(12): 1764-1774. doi: 10.1094/PDIS-02-15-0144-RE
Wang H, Yang XB, Ma Z (2010) Long-Distance Spore Transport of Wheat Stripe Rust Pathogen from Sichuan, Yunnan, and Guizhou in Southwestern China. Plant Disease 94(7): 873-880. doi: 10.1094/PDIS-94-7-0873
Wang et al. (2010) Characterization of a pathogenesis-related thaumatin-like protein gene TaPR5 from wheat induced by stripe rust fungus. Physiologia Plantarum 139(1): 27–38. doi: 10.1111/j.1399-3054.2009.01338.x
Wang X, Yang B, Li K, et al. (2016) A Conserved Puccinia striiformis Protein Interacts with Wheat NPR1 and Reduces Induction of Pathogenesis-Related Genes in Response to Pathogens. Molecular Plant-Microbe Interactions 29(12): 977-989. doi: 10.1094/MPMI-10-16-0207-R
Wang Z, Zhao J, Chen X, et al. (2016) Virulence Variations of Puccinia striiformis f. sp. tritici Isolates Collected from Berberis spp. in China. Plant Disease 100(1): 131-138. doi: 10.1094/PDIS-12-14-1296-RE
Wang J, Wang Y, Liu X, et al. (2016) Microtubule Polymerization Functions in Hypersensitive Response and Accumulation of H2O2 in Wheat Induced by the Stripe Rust. BioMed Research International vol. 2016, Article ID 7830768, 7 pages. doi: 10.1155/2016/7830768
Wang J, Tao F, An F, et al. (2017) Wheat transcription factor TaWRKY70 is positively involved in high‐temperature seedling plant resistance to Puccinia striiformis f. sp. tritici. Molecular Plant Pathology 18: 649-661. doi: 10.1111/mpp.12425
Wang J, Tao F, Tian W, et al. (2017) The wheat WRKY transcription factors TaWRKY49 and TaWRKY62 confer differential high-temperature seedling-plant resistance to Puccinia striiformis f. sp. tritici. PLoS ONE 12(7): e0181963. doi: 10.1371/journal.pone.0181963
Wang B, Sun Y, Song N, et al. (2017) Puccinia striiformis f. sp. tritici microRNA-like RNA 1 (Pst-milR1), an important pathogenicity factor of Pst, impairs wheat resistance to Pst by suppressing the wheat pathogenesis-related 2 gene. New Phytologist 215: 338–350. doi: 10.1111/nph.14577
Wang X, Wang Y, Liu P, et al. (2017) TaRar1 Is Involved in Wheat Defense against Stripe Rust Pathogen Mediated by YrSu. Frontiers in Plant Science 8:156. doi: 10.3389/fpls.2017.00156
Wang J, Wang J, Shang H, et al. (2019) TaXa21, a Leucine-Rich Repeat Receptor–Like Kinase Gene Associated with TaWRKY76 and TaWRKY62, Plays Positive Roles in Wheat High-Temperature Seedling Plant Resistance to Puccinia striiformis f. sp. tritici. Molecular Plant-Microbe Interactions 2019 32:1526-1535. https://doi.org/10.1094/MPMI-05-19-0137-R
Wang M, Wan A, Chen X (2022) Race Characterization of Puccinia striiformis f. sp. tritici in the United States from 2013 to 2017. Plant Disease. doi: 10.1094/PDIS-11-21-2499-RE
Wang N, Tang C, Fan X, et al. (2022) Inactivation of a wheat protein kinase gene confers broad-spectrum resistance to rust fungi. Cell 185: P2961-2974.E19. doi: 10.1016/j.cell.2022.06.027
Wang Y, Liu C, Du Y, et al. (2022) A stripe rust fungal effector PstSIE1 targets TaSGT1 to facilitate pathogen infection. Plant J, 112: 1413-1428. doi: 10.1111/tpj.16019
, , , et al. (2024) Abscisic acid-, stress-, ripening-induced 2 like protein, TaASR2L, promotes wheat resistance to stripe rust. Molecular Plant Pathology 25: e70028. doi: 10.1111/mpp.70028
Wellings CR, McIntosh RA, Walker J (1987) Puccinia striiformis f. sp. tritici in eastern Australia-possible means of entry and implications for plant quarantine. Plant Pathology 36: 239–241. doi: 10.1111/j.1365-3059.1987.tb02230.x
Wellings CR, Wright DG, Keiper F, Loughman R (2003) First detection of wheat stripe rust in Western Australia: evidence for a foreign incursion. Australasian Plant Pathology 32(2): 321–322. doi: 10.1071/AP03023
Wellings CR (2007) Puccinia striiformis in Australia: a review of the incursion, evolution, and adaptation of stripe rust in the period 1979–2006. Australian Journal of Agricultural Research 58: 567–575. doi: 10.1071/AR07130
Whetton RL, Hassall KL, Waine TW, Mouazen AM (2018) Hyperspectral measurements of yellow rust and fusarium head blight in cereal crops: Part 1: Laboratory study. Biosystems Engineering 166: 101-115. doi: 10.1016/j.biosystemseng.2018.01.004
Wu JH, Wang QL, Chen XM, et al. (2016) Stripe rust resistance in wheat breeding lines developed for central Shaanxi, an overwintering region for Puccinia striiformis f. sp. tritici in China. Canadian Journal of Plant Pathology 38(3): 317-324. doi: 10.1080/07060661.2016.1206039
Wu J, Wang Q, Liu S, et al. (2017) Saturation Mapping of a Major Effect QTL for Stripe Rust Resistance on Wheat Chromosome 2B in Cultivar Napo 63 Using SNP Genotyping Arrays. Front. Plant Sci. 8:653. doi: 10.3389/fpls.2017.00653
Wu J, Wang Q, Xu L, et al. (2017) Combining SNP genotyping array with bulked segregant analysis to map a gene controlling adult-plant resistance to stripe rust in wheat line 03031-1-5 H62. Phytopathology (accepted). doi: 10.1094/PHYTO-04-17-0153-R
Wu N, Ozketen AC, Cheng Y, et al. (2022) Puccinia striiformis f. sp. tritici effectors in wheat immune responses. Front. Plant Sci. 13: 1012216. doi: 10.3389/fpls.2022.1012216
Xia CJ, Wang MN, Wan AM, et al. (2016) Association Analysis of SP-SNPs and Avirulence Genes in Puccinia striiformis f. sp. tritici, the Wheat Stripe Rust Pathogen. American Journal of Plant Sciences 7: 126-137. doi: 10.4236/ajps.2016.71014
Xia C, Wang M, Yin C, et al. (2018) Genomic insights into host adaptation between the wheat stripe rust pathogen (Puccinia striiformis f. sp. tritici) and the barley stripe rust pathogen (Puccinia striiformis f. sp. hordei). BMC Genomics 19:664. doi: 10.1186/s12864-018-5041-y
Xia C, Qiu A, Wang M, et al. (2022) Current Status and Future Perspectives of Genomics Research in the Rust Fungi. International Journal of Molecular Sciences 23(17): 9629. doi: 10.3390/ijms23179629
Xiao M, Chen D, Liu S, et al. (2023) A chitin deacetylase PsCDA2 from Puccinia striiformis f. sp. tritici confers disease pathogenicity by suppressing chitin-triggered immunity in wheat. Molecular Plant Pathology 24: 1467–1479. doi: 10.1111/mpp.13381
Xing Y, Xia C, Huang L, et al. (2023) Population genomic analyses reveal extensive genomic regions within selective sweeps associated with adaptation and demographic history of a wheat fungal pathogen. bioRxiv 2023.08.29.555254; doi: 10.1101/2023.08.29.555254
Xu X, Ma LJ, Hu X (2018) Overwintering of Wheat Stripe Rust under Field Conditions in the Northwestern Regions of China. Plant Disease (accepted). doi: 10.1094/PDIS-06-18-1053-RE
Xu Q, Tang C, Wang L, et al. (2020) Haustoria – arsenals during the interaction between wheat and Puccinia striiformis f. sp. tritici. Molecular Plant Pathology 21: 83-94. doi: 10.1111/mpp.12882
, , , (2022) Detection method of wheat rust based on transfer learning and sharpness-aware minimization. Plant Pathology: 1– 8. doi: 10.1111/ppa.13661
Yan Q, Jia G, Tan W, et al. (2023) Genome-wide QTL mapping for stripe rust resistance in spring wheat line PI 660122 using the Wheat 15K SNP array. Front. Plant Sci. 14: 1232897. doi: 10.3389/fpls.2023.1232897
Yang Y, Chen F, Han D, et al. (2017) Evaluation of resistance of current wheat cultivars and breeding lines to stripe rust from three Gorges reservoir area. Journal of General Plant Pathology 83(5): 283-290. doi: 10.1007/s10327-017-0729-4
Yao Q, He MM, Hou L, et al. (2017) Genetic analysis and molecular mapping of stripe rust resistance genes in Chinese native wheat (Triticum aestivum) Lankao 5. Australasian Plant Pathology 46(3): 213-221. doi: 10.1007/s13313-017-0478-z
Yao F, Guan F, Duan L, et al. (2021) Genome-Wide Association Analysis of Stable Stripe Rust Resistance Loci in a Chinese Wheat Landrace Panel Using the 660K SNP Array. Front. Plant Sci. 12:783830. doi: 10.3389/fpls.2021.783830
Yao F, Wang M, See DR, et al. (2025) Identification of 39 stripe rust resistance loci in a panel of 465 winter wheat entries presumed to have high-temperature adult-plant resistance through genome-wide association mapping and marker-assisted detection. Front. Plant Sci. 15: 1514926. doi: 10.3389/fpls.2024.1514926
Yu M, Zhang H, Zhou XL, et al. (2017) Quantitative trait loci associated with agronomic traits and stripe rust in winter wheat mapping population using single nucleotide polymorphic markers. Molecular Breeding 37:105. doi: 10.1007/s11032-017-0704-y
Zakaria WGE, Atia MM, Ali AZ, et al. (2023) Assessing the Effectiveness of Eco-Friendly Management Approaches for Controlling Wheat Yellow Rust and Their Impact on Antioxidant Enzymes. Plants 12(16): 2954. doi: 10.3390/plants12162954
Zakeri A, Zakeri A, Afshari F, et al. (2016) Genetic analysis of resistance to stripe rust in some Iranian bread wheat cultivars and elite lines. Crop Breeding 6(2): 1-8. doi: 10.22092/CBJ.2016.107102
Zeng QD, Han DJ, Wang QL, et al. (2014) Stripe rust resistance and genes in Chinese wheat cultivars and breeding lines. Euphytica 196: 271-284. doi: 10.1007/s10681-013-1030-z
Zhan G, Ji F, Zhao J, et al. (2022) Sensitivity and Resistance Risk Assessment of Puccinia striiformis f. sp. tritici to Triadimefon in China. Plant Dis. 106(6): 1690-1699. doi: 10.1094/PDIS-10-21-2168-RE
Zhan G, Ji F, Chen X, et al. (2022) Populations of Puccinia striiformis f. sp. tritici in Winter Spore Production Regions Spread from Southwestern Oversummering Areas in China. Plant Disease PDIS09212070RE. doi: 10.1094/PDIS-09-21-2070-RE
Zhang Y, Liang X, Zhao M, et al. (2022) A novel ambigrammatic mycovirus, PsV5, works hand in glove with wheat stripe rust fungus to facilitate infection. Plant Communications 100505. doi: 10.1016/j.xplc.2022.100505
Zhang P, Lan C, Singh RP, et al. (2022) Identification and Characterization of Resistance Loci to Wheat Leaf Rust and Stripe Rust in Afghan Landrace “KU3067”. Front. Plant Sci. 13: 894528. doi: 10.3389/fpls.2022.894528
Zhang J, Li Y, Liu D, et al. (2025) Re-delimiting oversummering regions of Puccinia striiformis f. sp. tritici in China. Phytopathol Res 7: 13. doi: 10.1186/s42483-024-00308-y
Zhang M, Fang T, Zhou X, et al. (2022) Combination of Marker-Assisted Backcross Selection of Yr59 and Phenotypic Selection to Improve Stripe Rust Resistance and Agronomic Performance in Four Elite Wheat Cultivars. Agronomy 12(2): 497. doi: 10.3390/agronomy12020497
Zhao J, Wang M, Chen X, Kang Z (2016) Role of Alternate Hosts in Epidemiology and Pathogen Variation of Cereal Rusts. Annual Review of Phytopathology 54: 207-228. doi: 10.1146/annurev-phyto-080615-095851
Zheng W, Huang L, Huang J, et al. (2013) High genome heterozygosity and endemic genetic recombination in the wheat stripe rust fungus. Nature Communications 4: 2673. doi: 10.1038/ncomms3673
Zheng Q, Ye H, Huang W, et al. (2021) Integrating Spectral Information and Meteorological Data to Monitor Wheat Yellow Rust at a Regional Scale: A Case Study. Remote Sensing 13(2): 278. doi: 10.3390/rs13020278
Zheng X, Zhou J, Zhang M, et al. (2022) Transfer of Durable Stripe Rust Resistance Gene Yr39 into Four Chinese Elite Wheat Cultivars Using Marker-Assisted Selection. Agronomy 12(8): 1791. doi: 10.3390/agronomy12081791
Zhou X, Fang T, Li K, et al. (2022) Yield Losses Associated with Different Levels of Stripe Rust Resistance of Commercial Wheat Cultivars in China. Phytopathology 112(6): 1244-1254. doi: 10.1094/PHYTO-07-21-0286-R
Zhou A, Feng Y, Gao X, et al. (2023) Characterization of the triadimefon resistant Puccinia striiformis f. sp. tritici isolates in China. Phytopathol Res 5: 49. 10.1186/s42483-023-00205-w
Zhu X, Liu W, Chu X, et al. (2017) The transcription factor PstSTE12 is required for virulence of Puccinia striiformis f. sp. tritici. Molecular Plant Pathology. Accepted Author Manuscript. doi: 10.1111/mpp.12582
Župunski V, Savva L, Saunders DGO, Jevtić R (2024) A recent shift in the Puccinia striiformis f. sp. tritici population in Serbia coincides with changes in yield losses of commercial winter wheat varieties. Front. Plant Sci. 15: 1464454. doi: 10.3389/fpls.2024.1464454
.
.