.
Condición fitosanitaria: Presente
Grupo de cultivos: Frutícolas
Especie hospedante: Arándano (Vaccinium corymbosum L. 1753)
Rango de hospedantes: no específico / amplio. B. cinerea es un hongo polífago con un amplio rango de hospedantes y de amplia difusión mundial, siendo el agente causal de la podredumbre gris en diversos cultivos de importancia económica, tales como el arándano, la vid, el kiwi, la frutilla, el tomate, etc. Se han reportado más de 1400 especies de plantas atacadas por Botrytis, de 596 géneros, en 170 familias (Fillinger & Elad, 2016).
Epidemiología: policíclica, subaguda
Etiología: Hongo. Necrotrófico
Agente causal: Botrytis cinerea Pers.:Fr. (anamórfo), Botryotinia fuckeliana (de Bary) Whetzel (teleomorfo)
Taxonomía: Eukaryota > Fungi > Dikarya > Ascomycota > Pezizomycotina > Leotiomycetes > Helotiales > Sclerotiniaceae > Botrytis
.
- Cuerpos de fructificación sexual (apotecio), a partir de estructura de resistencia (esclerocio), del teleomorfo Botryotinia fuckeliana. Autor: Dean et al., 2012.
.
.
Síntomas
La infección generalmente se inicia en racimos florales u hojas, produciéndose una lesión circular, de color café y con forma circular. También puede afectar brotes nuevos. Los síntomas de la flor consistieron en una necrosis parcial a completa de los tejidos del cáliz y de la corola, con un pardeamiento necrótico en la superficie del fruto que eventualmente es acompañado por una piel resbaladiza y una abundante esporulación gris.
.
Esquema del ciclo de vida del patógeno
.
Daños
B. cinerea es uno de los hongos más frecuentes que atacan en postcosecha.
.
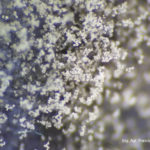
- 01 Botrytis cinerea en Arandano
- 02 Botrytis cinerea en Arandano
- 03 Botrytis cinerea en Arandano
- 04 Botrytis cinerea en Arandano
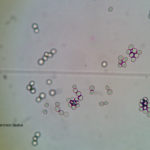
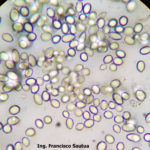
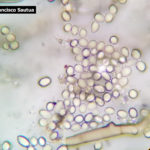
- 05 Conidios y conidióforos de Botrytis cinerea en Arandano
- 06 Conidióforo con abundantes conidios de Botrytis cinerea en Arandano
- 07 Conidios de Botrytis cinerea en Arandano
- 08 Conidios de Botrytis cinerea en Arandano
- 01 Botrytis cinerea en arándano
- 02 Botrytis cinerea en arándano
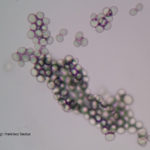
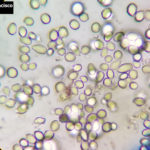
- 03 Conidios de Botrytis cinerea en arándano
- 04 Conidios de Botrytis cinerea en arándano
- 05 Botrytis cinerea en arándano
- 06 Botrytis cinerea en arándano
- 07 Conidios de Botrytis cinerea en arándano
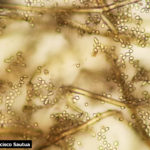
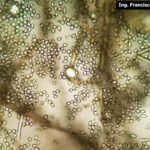
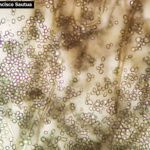
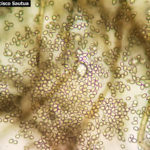
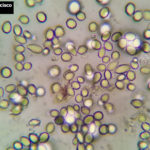
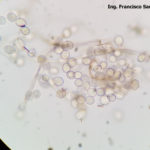
- 01 Conidios y conidióforos de Botrytis cinerea aislado de arándanos.
- 02 Conidios y conidióforos de Botrytis cinerea aislado de arándanos.
- 03 Conidios y conidióforos de Botrytis cinerea aislado de arándanos.
- 04 Conidios y conidióforos de Botrytis cinerea aislado de arándanos.
- 05 Conidios y conidióforos de Botrytis cinerea aislado de arándanos.
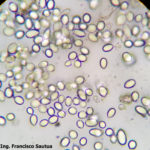
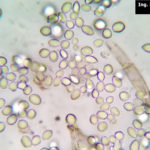
- 06 Conidios de Botrytis cinerea aislado de arándanos.
- 07 Conidios de Botrytis cinerea aislado de arándanos.
- 08 Conidios de Botrytis cinerea aislado de arándanos.
- 09 Conidios de Botrytis cinerea aislado de arándanos.
- 10 Conidios de Botrytis cinerea aislado de arándanos.
- 11 Conidios de Botrytis cinerea aislado de arándanos.
- 12 Conidios de Botrytis cinerea aislado de arándanos.
.
Epidemiología y condiciones predisponentes
El patógeno inverna como esclerocios o micelio en restos de frutas enfermas. En general el moho gris (Botryotinia fuckeliana / Botrytis cinerea) se considera un patógeno débil, que solo infecta las plantas dañadas o débiles. En primavera los esclerocios germinan, produciendo ramilletes de conidióforos de color negro, con los ápices llenos de conidios de color gris plomizo. La esporulación y dispersión de conidios (inóculo) es clave. La dispersión de los conidios es por viento. El proceso de infección requiere de agua libre sobre la superficie del follaje del arándano. Las lluvias con temperaturas frescas alrededor de la floración son las principales condiciones climáticas para el inicio de la enfermedad. La secreción de enzimas (cutinasa, pectinasas, etc.) y toxinas (botrydal) juegan también un rol clave en la patogenia. Infecciones latentes pueden mostrar síntomas y signos en postcosecha.
.
Manejo de la enfermedad
* Uso de variedades menos susceptibles.
* Debido a la gran cantidad de restos florales, que son potenciales fuentes de inóculo y de difícil eliminación, el manejo se basa preferentemente en el control químico, mediante el uso de fungicidas aplicados en forma preventiva desde pre-floración hasta cosecha. Los períodos críticos de control son la floración y la madurez de la fruta.
* Se deben respetar todas las buenas prácticas de manejo de fungicidas ya que B. cinerea es un patógeno con un alto nivel de variabilidad genética y de alto riesgo de generar resistencia a fungicidas.
* Evitar la fertilización excesiva de nitrógeno. Se debe lograr una fertilización balanceada.
* Manejo cultural: cortar y remover tejidos viejos infectados.
* Evitar cosechar frota sobremadura.
* No cosechar fruta mojada.
.
.
Bibliografía
Botrytis cinerea. Sistema Nacional Argentino de Vigilancia y Monitoreo de plagas
Bell SR, Montiel LGH, Estrada RRG, Martínez PG (2021) Main diseases in postharvest blueberries, conventional and eco-friendly control methods: A review. LWT 112046. doi: 10.1016/j.lwt.2021.112046
Amselem J, Cuomo CA, van Kan JAL, Viaud M, Benito EP, et al. (2011) Genomic Analysis of the Necrotrophic Fungal Pathogens Sclerotinia sclerotiorum and Botrytis cinerea. PLoS Genet 7(8): e1002230. doi: 10.1371/journal.pgen.1002230
Bi K, Scalschi L, Jaiswal N, et al. (2021) The Botrytis cinerea Crh1 transglycosylase is a cytoplasmic effector triggering plant cell death and defense response. Nature Communications 12: 2166. doi: 10.1038/s41467-021-22436-1
Cai Q, Qiao L, Wang M, He B, Lin FM, Palmquist J, Huang HD, Jin H (2018) Plants send small RNAs in extracellular vesicles to fungal pathogen to silence virulence genes. Science 17 May 2018: eaar4142. DOI: 10.1126/science.aar4142
Cheon W, Kim YS, Balaraju K, Kim BS, Lee BH, Jeon Y (2016) Postharvest Control of Botrytis cinerea and Monilinia fructigena in Apples by Gamma Irradiation Combined with Fumigation. Journal of Food Protection 79(8): 1410-1417. doi: 10.4315/0362-028X.JFP-15-532
Cheung N, Tian L, Liu X, Li X (2020) The Destructive Fungal Pathogen Botrytis cinerea—Insights from Genes Studied with Mutant Analysis. Pathogens 9(11): 923. doi: 10.3390/pathogens9110923
Dean R, Van Kan JAL, Pretorius ZA, Hammond-Kosack KE, Di Pietro A, Spanu PD, et al. (2012) The Top 10 fungal pathogens in molecular plant pathology. Molecular Plant Pathology 13: 414–430. doi: 10.1111/j.1364-3703.2011.00783.x
Duan Y, Yang Y, Wang JX, Chen C, Steinberg G, Fraaije B, Zhou M (2018) Simultaneous detection of multiple benzimidazole resistant β-tubulin variants of Botrytis cinerea using loop mediated isothermal amplification. Plant Disease (accepted). doi: 10.1094/PDIS-03-18-0542-RE
Emmanuel CJ, van Kan JA, Shaw MW (2018) Differences in the gene transcription state of Botrytis cinerea between necrotic and symptomless infections of lettuce and Arabidopsis thaliana. Plant Pathology doi: 10.1111/ppa.12907
Fillinger S, Elad Y (2016) Botrytis – the Fungus, the Pathogen and its Management in Agricultural Systems. Springer International Publishing Switzerland. doi: 10.1007/978-3-319-23371-0
Frías M, González C, Brito N (2011) BcSpl1, a cerato-platanin family protein, contributes to Botrytis cinerea virulence and elicits the hypersensitive response in the host. New Phytologist 192: 483–495. doi: 10.1111/j.1469-8137.2011.03802.x
Garfinkel AR (2021) The History of Botrytis Taxonomy, the Rise of Phylogenetics, and Implications for Species Recognition. Phytopathology 111(3): 437-454. doi: 10.1094/PHYTO-06-20-0211-IA
Gebremichael DE, Ciofini A, Sabbadini S, et al. (2025) Exogenous dsRNAs against chitin synthase and glucan synthase genes suppress the virulence of the pathogenic fungus Botrytis cinerea. J Plant Pathol 107: 251–264. doi: 10.1007/s42161-024-01812-y
Gupta R, Anand G, Pizarro L, et al. (2021) Cytokinin Inhibits Fungal Development and Virulence by Targeting the Cytoskeleton and Cellular Trafficking. ASM Journals mBio 12. doi: 10.1128/mBio.03068-20
Hahn M (2014) The rising threat of fungicide resistance in plant pathogenic fungi: Botrytis as a case study. Journal of Chemical Biology 7: 133–141. doi: 10.1007/s12154-014-0113-1
Hahn M, Viaud M, van Kan J (2014) The Genome of Botrytis cinerea, a Ubiquitous Broad Host Range Necrotroph. In: R. A. Dean et al. (eds.), Genomics of Plant-Associated Fungi and Oomycetes: Dicot Pathogens, Springer-Verlag Berlin Heidelberg 2014. doi: 10.1007/978-3-662-44056-8_2
Hu MJ, Cox KD, Schnabel G (2016) Resistance to Increasing Chemical Classes of Fungicides by Virtue of “Selection by Association” in Botrytis cinerea. Phytopathology 106(12): 1513-1520. doi: 10.1094/PHYTO-04-16-0161-R
Izquierdo-Bueno I, González-Rodríguez VE, Simon A, et al. (2018) Biosynthesis of abscisic acid in fungi: identification of a sesquiterpene cyclase as the key enzyme in Botrytis cinerea. Environ Microbiol. 20(7): 2469-2482. doi: 10.1111/1462-2920.14258
Jiang M, Xu X, Song J, et al. (2021) Streptomyces botrytidirepellens sp. nov., a novel actinomycete with antifungal activity against Botrytis cinerea. Int J Syst Evol Microbiol. 71(9). doi:
Kirschbaum DS, Alderete GL, Rivadeneira M, et al. (2015) Reconocimiento de plagas, organismos benéficos y enfermedades habituales del cultivo de frutillas en en noroeste argentino. Guía práctica de campo. Editor/es: Min. de Producción, SAF, Delegación Jujuy. INTA. PRODERI.. – Página/s: 20.
Kretschmer M, Leroch M, Mosbach A, et al. (2009) Fungicide-Driven Evolution and Molecular Basis of Multidrug Resistance in Field Populations of the Grey Mould Fungus Botrytis cinerea. PLoS Pathog 5(12): e1000696. doi: 10.1371/journal.ppat.1000696
Kumari S, Tayal P, Sharma E, Kapoor R (2014) Analyses of genetic and pathogenic variability among Botrytis cinerea isolates. Microbiological Research 169: 862-872. doi: 10.1016/j.micres.2014.02.012
Kuroyanagi T, Bulasag AS, Fukushima K, et al. (2022) Botrytis cinerea identifies host plants via the recognition of antifungal capsidiol to induce expression of a specific detoxification gene. PNAS Nexus 1(5): pgac274. doi: 10.1093/pnasnexus/pgac274
Kwon JH, Cheon MG, Choi O, Kim J (2011) First Report of Botrytis cinerea as a Postharvest Pathogen of Blueberry in Korea. Mycobiology 39(1): 52-3. doi: 10.4489/MYCO.2011.39.1.052
Leisen T, Werner J, Pattar P, et al. (2022) Multiple knockout mutants reveal a high redundancy of phytotoxic compounds contributing to necrotrophic pathogenesis of Botrytis cinerea. PLoS Pathog 18(3): e1010367. doi: 10.1371/journal.ppat.1010367
Li X, Gao X, Hu S, et al. (2022) Resistance to pydiflumetofen in Botrytis cinerea: risk assessment and detection of point mutations in sdh genes that confer resistance. Pest Manag Sci 78: 1448-1456. doi: 10.1002/ps.6762
Li X, Yang J, Jiang Q, et al. (2022) Baseline sensitivity and control efficacy of a new QiI fungicide, florylpicoxamid, against Botrytis cinerea. Pest Manag Sci. 78: 5184-5190. doi: 10.1002/ps.7137
Liu Y, Liu JK, Li GH, et al. (2019) A novel Botrytis cinerea-specific gene BcHBF1 enhances virulence of the grey mould fungus via promoting host penetration and invasive hyphal development. Molecular Plant Pathology 20(5): 731-747. doi: 10.1111/mpp.12788
Liu K, Wen Z, Ma Z, et al. (2022) Biological and molecular characterizations of fluxapyroxad-resistant isolates of Botrytis cinerea. Phytopathol Res 4: 2. doi: 10.1186/s42483-022-00107-3
López Ortega MP (2012) Control Biológico de Botrytis sp. mediante levaduras filosféricas en rosas de corte tipo exportación. Tesis de Maestria, Universidad Nacional de Colombia. 111 pp.
Malandrakis AA, Krasagakis N, Kavroulakis N (2022) Fungicide resistance frequencies of Botrytis cinerea greenhouse isolates and molecular detection of a novel SDHI resistance mutation. Pesticide Biochemistry and Physiology: 105058. doi: 10.1016/j.pestbp.2022.105058
Maridueña-Zavala MG, Freire-Peñaherrera A, Cevallos-Cevallos JM, et al. (2017) GC-MS metabolite profiling of Phytophthora infestans resistant to metalaxyl. European Journal of Plant Pathology 149(3): 563-574. doi: 10.1007/s10658-017-1204-y
Mbengue M, Navaud O, Peyraud R, Barascud M, Badet T, Vincent R, Barbacci A and Raffaele S (2016) Emerging Trends in Molecular Interactions between Plants and the Broad Host Range Fungal Pathogens Botrytis cinerea and Sclerotinia sclerotiorum. Frontiers in Plant Science 7: 422. doi: 10.3389/fpls.2016.00422
McClellan WD, Hewitt WB (1973) Early Botrytis Rot of Grapes: Time of Infection and Latency of Botrytis cinerea Pers. in Vitis vinifera L. Phytopathology 63:1151-1157. doi: 10.1094/Phyto-63-1151
Meng L, Mestdagh H, Ameye M, et al. (2020) Phenotypic Variation of Botrytis cinerea Isolates Is Influenced by Spectral Light Quality. Front. Plant Sci. 11: 1233. doi: 10.3389/fpls.2020.01233
Müller N, Leroch M, Schumacher J, et al. (2018) Investigations on VELVET regulatory mutants confirm the role of host tissue acidification and secretion of proteins in the pathogenesis of Botrytis cinerea. New Phytol, 219: 1062-1074. doi: 10.1111/nph.15221
Noda J, Brito N, Gonzalez C (2010) The Botrytis cinerea xylanase Xyn11A contributes to virulence with its necrotizing activity, not with its catalytic activity. BMC Plant Biol 10: 38. doi: 10.1186/1471-2229-10-38
Oren-Young L, Llorens E, Bi K, Zhang M, Sharon A (2021) Botrytis cinerea methyl isocitrate lyase mediates oxidative stress tolerance and programmed cell death by modulating cellular succinate levels. Fungal Genetics and Biology 146: 103484. https://doi.org/10.1016/j.fgb.2020.103484
Plesken C, Pattar P, Reiss B, et al. (2021) Genetic Diversity of Botrytis cinerea Revealed by Multilocus Sequencing, and Identification of B. cinerea Populations Showing Genetic Isolation and Distinct Host Adaptation. Front. Plant Sci. 12: 663027. doi: 10.3389/fpls.2021.663027
Richards JK, Xiao CL, Jurick WM 2nd (2021) Botrytis spp.: A Contemporary Perspective and Synthesis of Recent Scientific Developments of a Widespread Genus that Threatens Global Food Security. Phytopathology 111(3): 432-436. doi: 10.1094/PHYTO-10-20-0475-IA
Roca-Couso R, Flores-Félix JD, Rivas R (2021) Mechanisms of Action of Microbial Biocontrol Agents against Botrytis cinerea. Journal of Fungi. 7(12): 1045. doi: 10.3390/jof7121045
Rollins JA, Cuomo CA, Dickman MB, Kohn LM (2014) Genomics of Sclerotinia sclerotiorum. In: Dean R, Lichens-Park A, Kole C (eds) Genomics of Plant-Associated Fungi and Oomycetes: Dicot Pathogens. pp. 1-17. Springer, Berlin, Heidelberg. doi: 10.1007/978-3-662-44056-8_1
Rossi FR, Krapp AR, Bisaro F, Maiale SJ, Pieckenstain FL, Carrillo N (2017) Reactive oxygen species generated in chloroplasts contribute to tobacco leaf infection by the necrotrophic fungus Botrytis cinerea. Plant Journal (accepted). doi: 10.1111/tpj.13718
Sautua FJ, Baron C, Pérez-Hernández O, Carmona MA (2019) First report of resistance to carbendazim and procymidone in Botrytis cinerea from strawberry, blueberry and tomato in Argentina. Crop Protection 125: 104879. doi: 10.1016/j.cropro.2019.104879
Shah P, Gutierrez-Sanchez G, Orlando R, Bergmann C (2009) A proteomic study of pectin-degrading enzymes secreted by Botrytis cinerea grown in liquid culture. Proteomics 9: 3126–3135. doi: 10.1002/pmic.200800933
Schouten A, van Baarlen P, van Kan JAL (2008) Phytotoxic Nep1-like proteins from the necrotrophic fungus Botrytis cinerea associate with membranes and the nucleus of plant cells. New Phytologist, 177: 493–505. doi: 10.1111/j.1469-8137.2007.02274.x
, (2023) Genetic and molecular landscapes of the generalist phytopathogen Botrytis cinerea. Molecular Plant Pathology 00: 1–19. doi: 10.1111/mpp.13404
Sofianos G, Samaras A, Karaoglanidis G (2023) Multiple and multidrug resistance in Botrytis cinerea: molecular mechanisms of MLR/MDR strains in Greece and effects of co-existence of different resistance mechanisms on fungicide sensitivity. Front. Plant Sci. 14: 1273193. doi: 10.3389/fpls.2023.1273193
ten Have A, Mulder W, Visser J, van Kan JAL (1998) The endopoly- galacturonase gene Bcpg1 is required for full virulence of Botrytis cinerea. Molecular Plant-Microbe Interactions 11(10): 1009–1016. doi: 10.1094/MPMI.1998.11.10.1009
, , , (2023) Plant defensin MtDef4-derived antifungal peptide with multiple modes of action and potential as a bio-inspired fungicide. Molecular Plant Pathology 00: 1– 18. doi: 10.1111/mpp.13336
Tian X, Song L, Wang Y, Jin W, Tong F and Wu F (2018) miR394 Acts as a Negative Regulator of Arabidopsis Resistance to B. cinerea Infection by Targeting LCR. Front. Plant Sci. 9:903. doi: 10.3389/fpls.2018.00903
Tut G, Magan N, Brain P, Xu X (2021) Critical Evaluation of Two Commercial Biocontrol Agents for Their Efficacy against B. cinerea under In Vitro and In Vivo Conditions in Relation to Different Abiotic Factors. Agronomy 11(9):1868. doi: 10.3390/agronomy11091868
Valero-Jiménez CA, Steentjes MBF, Slot JC, Shi-Kunne X, Scholten OE, van Kan JAL (2020) Dynamics in Secondary Metabolite Gene Clusters in Otherwise Highly Syntenic and Stable Genomes in the Fungal Genus Botrytis. Genome Biology and Evolution 12(12): 2491-2507. doi: 10.1093/gbe/evaa218
Van Kan JAL, Stassen JHM, Mosbach A, Van Der Lee TAJ, Faino L, Farmer AD, Papasotiriou DG, Zhou S, Seidl MF, Cottam E, Edel D, Hahn M, Schwartz DC, Dietrich RA, Widdison S, Scalliet G (2017) A gapless genome sequence of the fungus Botrytis cinerea. Molecular Plant Pathology 18: 75–89. doi: 10.1111/mpp.12384
Veloukas T, Karaoglanidis GS (2012) Biological activity of the succinate dehydrogenase inhibitor fluopyram against Botrytis cinerea and fungal baseline sensitivity. Pest. Manag. Sci. 68: 858-864. doi: 10.1002/ps.3241
Wilkinson SW, Pastor V, Paplauskas S, Pétriacq P, Luna E (2018) Long-lasting β-aminobutyric acid-induced resistance protects tomato fruit against Botrytis cinerea. Plant Pathology 67: 30–41. doi: 10.1111/ppa.12725
Williamson B, Tudzynski B, Tudzynski P, Van Kan JAL (2007) Botrytis cinerea: the cause of grey mould disease. Molecular Plant Pathology 8(5): 561–580. doi: 10.1111/J.1364-3703.2007.00417.X
Yang R, Li N, Zhou Z, Li G (2021) Characterization of the Populations of Botrytis cinerea Infecting Plastic Tunnel-Grown Strawberry and Tomato in the Hubei Province of China. Plant Dis. 105(7): 1890-1897. doi: 10.1094/PDIS-01-20-0164-RE
Zhang ZQ, Qin GZ, Li BQ, Tian SP (2014) Knocking out Bcsas1 in Botrytis cinerea impacts growth, development, and secretion of extracellular proteins which decreases virulence. Molecular Plant-Microbe Interactions 27: 590–600. doi: 10.1094/MPMI-10-13-0314-R
, , , et al. (2020) Transcriptome analysis and functional validation reveal a novel gene, BcCGF1, that enhances fungal virulence by promoting infection‐related development and host penetration. Molecular Plant Pathology 21: 834– 853. doi: 10.1111/mpp.12934