.
Condición fitosanitaria: Presente
Agente causal: Aspergillus flavus Link 1809 Link, 1809, Aspergillus niger Tiegh.
Taxonomía: Fungi > Ascomycota > Pezizomycotina > Eurotiomycetes > Eurotiomycetidae > Eurotiales > Aspergillaceae > Aspergillus > Aspergillus subgen. Circumdati
.
.
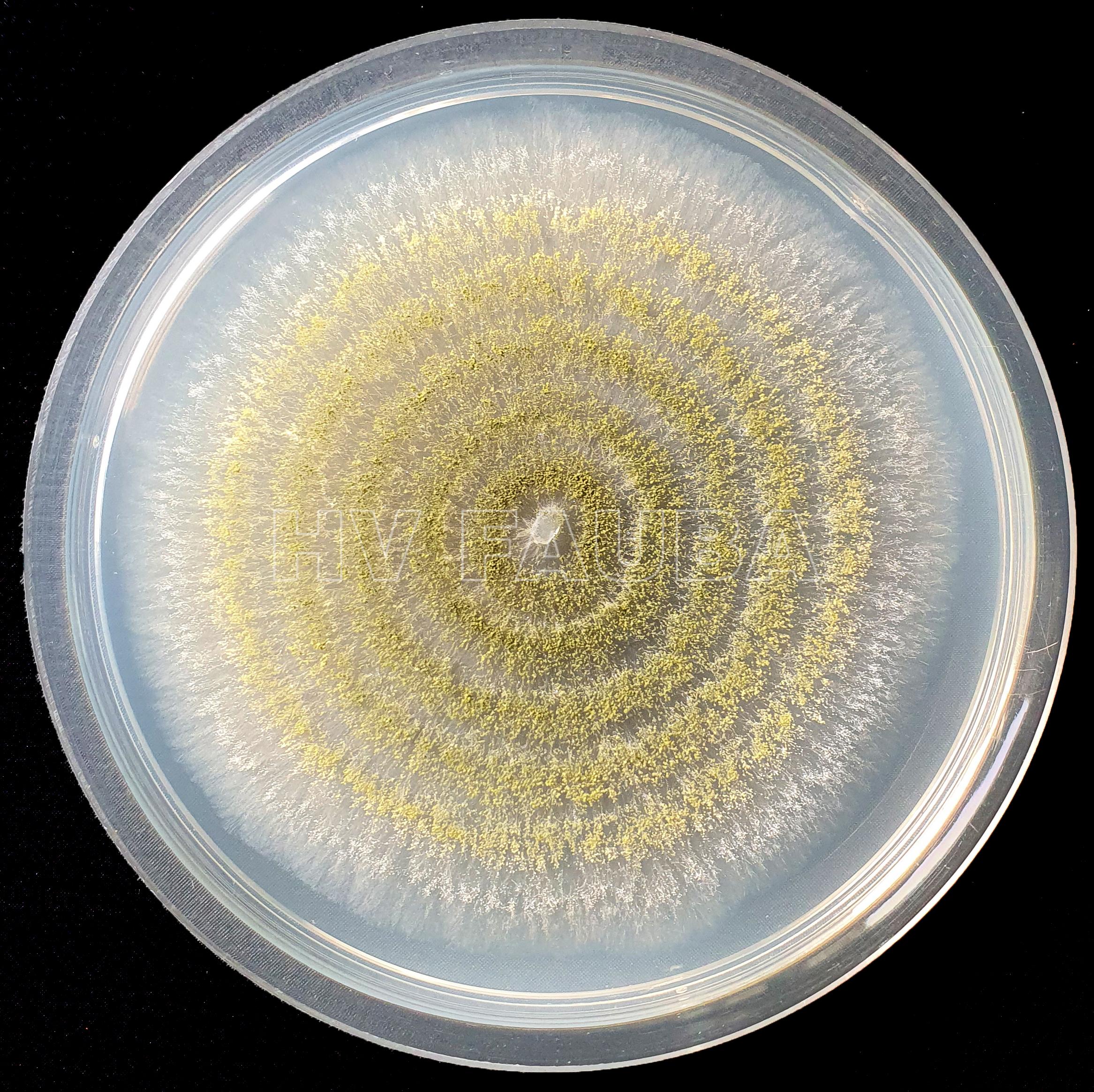
- Colonia de Aspergillus flavus en PDA. Autor: Dra Luján Cuestas.
- Colonia de Aspergillus flavus en PDA. Autor: Francisco Sautua
- Colonia de Aspergillus flavus en PDA. Autor: Francisco Sautua
- Colonia de Aspergillus niger creciendo en APD. Autores: Francisco Sautua y Marcelo Carmona.
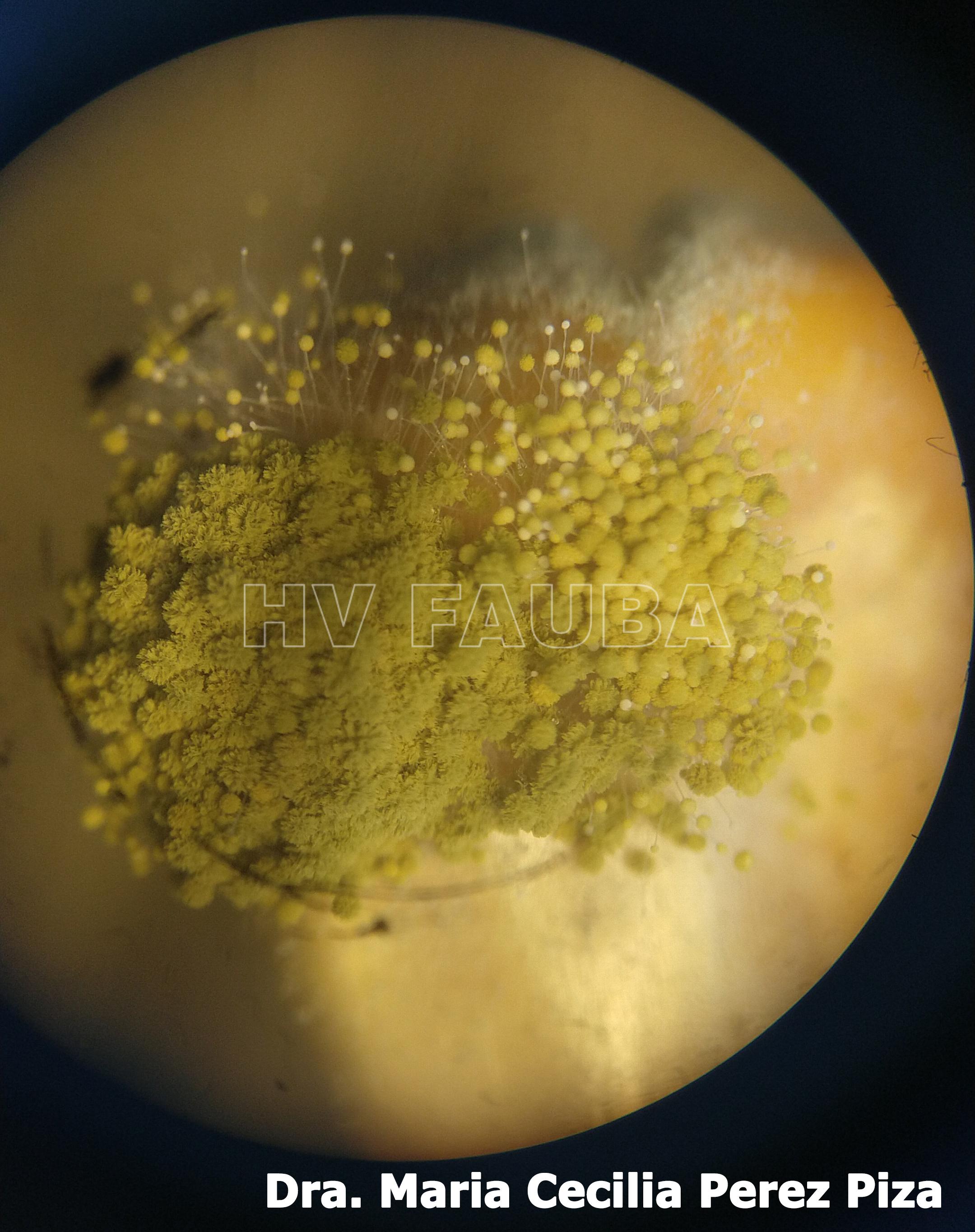
- Autor: Dra. Maria Cecilia Perez Piza
- Autor: Dra. Maria Cecilia Perez Piza
- Autor: Dra. Maria Cecilia Perez Piza
.
.
.
- Autor: Ed Sikora
.

Grano de soja colonizado por Aspergillus flavus. Autores: Dr. Francisco Sautua, Dr Marcelo Carmona
.
Bibliografía
Ahangarkani F, Badali H, Abbasi K, et al. (2020) Clonal Expansion of Environmental Triazole Resistant Aspergillus fumigatus in Iran. Journal of Fungi. 6(4): 199. doi: 10.3390/jof6040199
Ajmal M, Alshannaq AF, Moon H, et al. (2022) Characterization of 260 Isolates of Aspergillus Section Flavi Obtained from Sesame Seeds in Punjab, Pakistan. Toxins 14(2): 117. doi: 10.3390/toxins14020117
Annick Ries LN, Alves de Castro P, Pereira Silva L (2021) Aspergillus fumigatus Acetate Utilization Impacts Virulence Traits and Pathogenicity. mBio 12. doi: 10.1128/mBio.01682-21
Apud GR, Aredes-Fernández PA, Kritsanida M, et al. (2020) Antifungal activity of Bignoniaceae plants on Aspergillus carbonarius and Aspergillus niger. Nat Prod Res. 34(18): 2656-2659. doi: 10.1080/14786419.2018.1548453
Arastehfar A, Lass-Flörl C, Garcia-Rubio R, et al. (2020) The Quiet and Underappreciated Rise of Drug-Resistant Invasive Fungal Pathogens. Journal of Fungi 6(3): 138. doi: 10.3390/jof6030138
Barber AE, Riedel J, Sae-Ong T, et al. (2020) Effects of Agricultural Fungicide Use on Aspergillus fumigatus Abundance, Antifungal Susceptibility, and Population Structure. mBio. 11(6): e02213-20. doi: 10.1128/mBio.02213-20
Burks C, Darby A, Gómez Londoño L, et al. (2021) Azole-resistant Aspergillus fumigatus in the environment: Identifying key reservoirs and hotspots of antifungal resistance. PLoS Pathog 17(7): e1009711. doi: 10.1371/journal.ppat.1009711
, , , et al. (2021) Collateral consequences of agricultural fungicides on pathogenic yeasts: A One Health perspective to tackle azole resistance. Mycoses 00: 1– 9. doi:10.1111/myc.13404
(2024) Pan-azole- and multi-fungicide-resistant Aspergillus fumigatus is widespread in the United States. Appl Environ Microbiol 90: e01782-23. doi: 10.1128/aem.01782-23
Chulze SN, Tittlemier S, Torres AM (2023) Editorial: Fusarium species as plant and human pathogens, mycotoxin producers, and biotechnological importance. Front. Fungal Biol. 4: 1320198. doi: 10.3389/ffunb.2023.1320198
Colabardini AC, Wang F, Dong Z, et al. (2021) Heterogeneity in the transcriptional response of the human pathogen Aspergillus fumigatus to the antifungal agent caspofungin, Genetics iyab183. doi: 10.1093/genetics/iyab183
Colabardini AC, Van Rhijn N, LaBella AL, et al. (2022) Aspergillus fumigatus FhdA Transcription Factor Is Important for Mitochondrial Activity and Codon Usage Regulation during the Caspofungin Paradoxical Effect. Antimicrob Agents Chemother.: e0070122. doi: 10.1128/aac.00701-22
Del Palacio A, Bettucci L, Pan D (2016) Fusarium and Aspergillus mycotoxins contaminating wheat silage for dairy cattle feeding in Uruguay. Braz J Microbiol. 47(4): 1000-1005. doi: 10.1016/j.bjm.2016.06.004
Djenontin E, Debourgogne A, Mousavi B, et al. (2024) Azole resistance in Aspergillus flavus and associated fitness cost. Mycoses 67: e13766. doi:10.1111/myc.13766
Doughty KJ, Sierotzki H, Semar M, Goertz A (2021) Selection and Amplification of Fungicide Resistance in Aspergillus fumigatus in Relation to DMI Fungicide Use in Agronomic Settings: Hotspots versus Coldspots. Microorganisms 9(12): 2439. doi: 10.3390/microorganisms9122439
Dudakova A, Spiess B, Tangwattanachuleeporn M, et al. (2017) Molecular Tools for the Detection and Deduction of Azole Antifungal Drug Resistance Phenotypes in Aspergillus Species. Clin Microbiol Rev. 30(4): 1065-1091. doi: 10.1128/CMR.00095-16
Ekpakpale DO, Kraak B, Meijer M, et al. (2021) Fungal Diversity and Aflatoxins in Maize and Rice Grains and Cassava-Based Flour (Pupuru) from Ondo State, Nigeria. Journal of Fungi 7(8): 635. doi: 10.3390/jof7080635
Fraaije B, Atkins S, Hanley S, et al. (2020) The Multi-Fungicide Resistance Status of Aspergillus fumigatus Populations in Arable Soils and the Wider European Environment. Front. Microbiol. 11: 599233. doi: 10.3389/fmicb.2020.599233
Garcia-Effron G (2021) Molecular Markers of Antifungal Resistance: Potential Uses in Routine Practice and Future Perspectives. Journal of Fungi 7(3): 197. doi: 10.3390/jof7030197
Crossover between the control of fungal pathogens in medicine and the wider environment, and the threat of antifungal resistance. Plant Pathol. 00: 1– 19. doi: 10.1111/ppa.13429
Giusiano, G.E., Piontelli, E., Fernández, M.S., et al. (2016) Biodiversity of species of Aspergillus section Fumigati in semi-desert soils in Argentina. Revista Argentina de Microbiología 49: 247-254. doi: 10.1016/j.ram.2017.02.002
Gomez AA, Terán Baptista ZP, Mandova T, et al. (2020) Antifungal and antimycotoxigenic metabolites from native plants of northwest Argentina: isolation, identification and potential for control of Aspergillus species. Nat Prod Res. 34(22): 3299-3302. doi: 10.1080/14786419.2018.1560286
Guinea J (2020) Updated EUCAST Clinical Breakpoints against Aspergillus, Implications for the Clinical Microbiology Laboratory. Journal of Fungi 6(4): 343. doi: 10.3390/jof6040343
Haghani I, Yahyazadeh Z, Hedayati MT, et al. (2023) Antifungal activity of miltefosine against both azole-susceptible and -resistant Aspergillus strains. International Journal of Antimicrobial Agents 61: 106715. doi: 10.1016/j.ijantimicag.2023.106715
Hermida‐Alava K, Brito Devoto T, Sautua F, et al. (2021) Antifungal Susceptibility Profile and Molecular Identification of Cyp51C Mutations in Clinical and Environmental Isolates of Aspergillus flavus from Argentina. Mycoses 64: 95–101. doi: 10.1111/myc.13193
Horta MAC, Steenwyk JL, Mead ME, et al. (2022) Examination of Genome-Wide Ortholog Variation in Clinical and Environmental Isolates of the Fungal Pathogen Aspergillus fumigatus. mBio. 2022 Jun 29: e0151922. doi: 10.1128/mbio.01519-22
Jia LJ, Rafiq M, Radosa L, et al. (2022) Re-direction of phagosomes to the recycling expulsion pathway by a fungal pathogen. bioRxiv 2022.05.18.492126; doi: 10.1101/2022.05.18.492126
Jørgensen KM, Helleberg M, Hare RK, et al. (2021) Dissection of the Activity of Agricultural Fungicides against Clinical Aspergillus Isolates with and without Environmentally and Medically Induced Azole Resistance. Journal of Fungi 7(3): 205. doi: 10.3390/jof7030205
Kagot V, De Boevre M, De Saeger S, et al. (2022) Incidence of toxigenic Aspergillus and Fusarium species occurring in maize kernels from Kenyan households. World Mycotoxin Journal 15 (4): 407 – 416. doi: 10.3920/WMJ2021.2748
Kang SE, Sumabat LG, Melie T, et al. (2021) Evidence for the agricultural origin of resistance to multiple antimicrobials in Aspergillus fumigatus, a fungal pathogen of humans. G3 (Bethesda): jkab427. doi: 10.1093/g3journal/jkab427
Kun RS, Garrigues S, Di Falco M, Tsang A, de Vries RP (2021) Blocking utilization of major plant biomass polysaccharides leads Aspergillus niger towards utilization of minor components. Microb Biotechnol. doi: 10.1111/1751-7915.13835
Macedo D, Brito Devoto T, Pola S, et al. (2020) A Novel Combination of CYP51A Mutations Confers Pan-Azole Resistance in Aspergillus fumigatus. Antimicrob Agents Chemother. 64(8): e02501-19. doi: 10.1128/AAC.02501-19
Magnoli K, Benito N, Carranza C, et al. (2021) Effects of chlorpyrifos on growth and aflatoxin B1 production by Aspergillus section Flavi strains on maize-based medium and maize grains. Mycotoxin Res 37: 51–61. doi: 10.1007/s12550-020-00412-w
Maxwell LA, Callicott K, Bandyopadhyay R, et al. (2021) Degradation of aflatoxin B1 by atoxigenic Aspergillus flavus biocontrol agents. Plant Disease. doi: 10.1094/PDIS-01-21-0066-RE
McDermott A (2022) Drug-resistant fungi on the rise. PNAS 119 (48): e2217948119. doi: 10.1073/pnas.2217948119
Mohapatra D, Kumar S, Kotwaliwale N, Singh KK (2017) Critical factors responsible for fungi growth in stored food grains and non-Chemical approaches for their control. Industrial Crops and Products 108: 162-182. doi: 10.1016/j.indcrop.2017.06.039
Molinero RL, Hermida Alava KS, Brito Devoto B, et al. (2024) Prevalence of azole-resistant A. fumigatus and other aspergilli in the environment from Argentina. Medical Mycology; myae098. doi: 10.1093/mmy/myae098
Moral J, Garcia-Lopez MT, Gordon A, et al. (2022) Resistance to Aspergillus flavus and Aspergillus parasiticus in Almond Advanced Selections and Cultivars and Its Interaction with the Aflatoxin Biocontrol Strategy. Plant Disease 106(2): 504-509. doi: 10.1094/PDIS-05-21-0892-RE
Pérez-Cantero A, López-Fernández L, Guarro J, Capilla J (2020) Azole resistance mechanisms in Aspergillus: update and recent advances. International Journal of Antimicrobial Agents 55: 105807. doi: 10.1016/j.ijantimicag.2019.09.011
Perrone G, Susca A, Cozzi G, et al. (2007) Biodiversity of Aspergillus species in some important agricultural products. Stud Mycol. 59: 53-66. doi: 10.3114/sim.2007.59.07
Pfaller M, Boyken L, Hollis R, et al. (2011) Use of epidemiological cutoff values to examine 9-year trends in susceptibility of Aspergillus species to the triazoles. J Clin Microbiol. 49(2): 586-90. doi: 10.1128/JCM.02136-10
Pfliegler WP, Pócsi I, Győri Z, Pusztahelyi T (2020) The Aspergilli and Their Mycotoxins: Metabolic Interactions With Plants and the Soil Biota. Front. Microbiol. 10: 2921. doi: 10.3389/fmicb.2019.02921
Prigitano A, Venier V, Cogliati M, et al. (2014) Azole-resistant Aspergillus fumigatus in the environment of northern Italy, May 2011 to June 2012. Euro Surveill. 19(12): 20747. doi: 10.2807/1560-7917.es2014.19.12.20747
Rabiço F, Borelli TC, Alnoch RC, et al. (2024) Novel Pseudomonas Species Prevent the Growth of the Phytopathogenic Fungus Aspergillus flavus. BioTech. 13(2): 8. doi: 10.3390/biotech13020008
Resendiz-Sharpe A, Dewaele K, Merckx R, et al. (2021) Triazole-Resistance in Environmental Aspergillus fumigatus in Latin American and African Countries. J Fungi (Basel) 7(4): 292. doi: 10.3390/jof7040292
Rhodes J, Abdolrasouli A, Dunne K, et al. (2022) Population genomics confirms acquisition of drug-resistant Aspergillus fumigatus infection by humans from the environment. Nat Microbiol 7: 663–674. doi: 10.1038/s41564-022-01091-2
Riat A, Plojoux J, Gindro K, et al. (2018) Azole Resistance of Environmental and Clinical Aspergillus fumigatus Isolates from Switzerland. Antimicrob Agents Chemother. 62(4): e02088-17. doi: 10.1128/AAC.02088-17
Rocchi S, Godeau C, Crini G, Snelders E (2021) Emergence of a Pathogenic Fungus Resistant to Triazole Antifungal Drugs. In: Morin-Crini N, Lichtfouse E, Crini G (eds) Emerging Contaminants Vol. 1. Environmental Chemistry for a Sustainable World, vol 65. Springer, Cham. doi: 10.1007/978-3-030-69079-3_3
Schoustra SE, Debets AJM, Rijs AJMM, et al. (2019) Environmental Hotspots for Azole Resistance Selection of Aspergillus fumigatus, the Netherlands. Emerg Infect Dis. 25(7): 1347-1353. doi: 10.3201/eid2507.181625
Shishodia SK, Tiwari S, Shankar J (2019) Resistance mechanism and proteins in Aspergillus species against antifungal agents. Mycology 10(3): 151-165. doi: 10.1080/21501203.2019.1574927
Steffan BN, Venkatesh N, Keller NP (2020) Let’s Get Physical: Bacterial-Fungal Interactions and Their Consequences in Agriculture and Health. Journal of Fungi. 6(4):243. doi: 10.3390/jof6040243
Suzuki T, Iwahashi Y (2016) Addition of Carbon to the Culture Medium Improves the Detection Efficiency of Aflatoxin Synthetic Fungi. Toxins 8(11): 338. doi: 10.3390/toxins8110338
Testempasis SI, Karaoglanidis GS (2023) Resistance of Black Aspergilli Species from Grape Vineyards to SDHI, QoI, DMI, and Phenylpyrrole Fungicides. Journal of Fungi 9(2): 221. doi: 10.3390/jof9020221
Toda M, Beer KD, Kuivila KM, Chiller TM, Jackson BR (2021) Trends in Agricultural Triazole Fungicide Use in the United States, 1992-2016 and Possible Implications for Antifungal-Resistant Fungi in Human Disease. Environ Health Perspect. 129(5): 55001. doi: 10.1289/EHP7484
Valero C, Colabardini AC, de Castro PA, et al. (2021) Aspergillus Fumigatus ZnfA, a Novel Zinc Finger Transcription Factor Involved in Calcium Metabolism and Caspofungin Tolerance. Front. Fungal Biol. 2: 689900. doi: 10.3389/ffunb.2021.689900
Valero C, Pinzan CF, de Castro PA, et al. (2023) A phylogenetic approach to explore the Aspergillus fumigatus conidial surface-associated proteome and its role in pathogenesis. bioRxiv 2023.08.22.553365; doi: 10.1101/2023.08.22.553365
van der Torre MH, Novak-Frazer L, Rautemaa-Richardson R (2021) Detecting Azole-Antifungal Resistance in Aspergillus fumigatus by Pyrosequencing. Journal of Fungi 6(1):12. doi: 10.3390/jof6010012
Van Rhijn N, Bromley M, Richardson M, Bowyer P (2021) CYP51 Paralogue Structure Is Associated with Intrinsic Azole Resistance in Fungi. MBio 12. doi: 10.1128/mBio.01945-21
Verweij PE, Lucas JA, Arendrup MC, et al. (2020) The one health problem of azole resistance in Aspergillus fumigatus: current insights and future research agenda. Fungal Biology Reviews 34: 202-214. doi: 10.1016/j.fbr.2020.10.003
Wang F, Sethiya P, Hu X, et al. (2021) Transcription in fungal conidia before dormancy produces phenotypically variable conidia that maximize survival in different environments. Nat Microbiol 6: 1066–1081. doi: 10.1038/s41564-021-00922-y
Wang Y, Zhang L, Zhou L, et al. (2022) Epidemiology, Drug Susceptibility, and Clinical Risk Factors in Patients With Invasive Aspergillosis. Front. Public Health 10: 835092. doi: 10.3389/fpubh.2022.835092
Verweij PE, Arendrup MC, Alastruey-Izquierdo A, et al. (2022) Dual use of antifungals in medicine and agriculture: How do we help prevent resistance developing in human pathogens? Drug Resistance Updates 65: 100885. doi: 10.1016/j.drup.2022.100885
Xiong ZR, Cobo M, Whittal RM, et al. (2022) Purification and characterization of antifungal lipopeptide produced by Bacillus velezensis isolated from raw honey. PLoS ONE 17(4): e0266470. doi: 10.1371/journal.pone.0266470
Xu S, Shen J, Lang H, et al. (2022) Triazole resistance in Aspergillus fumigatus exposed to new chiral fungicide mefentrifluconazole. Pest Manag Sci. doi: 10.1002/ps.7224
Zhao S, Gibbons JG (2018) A population genomic characterization of copy number variation in the opportunistic fungal pathogen Aspergillus fumigatus. PLoS One 13(8): e0201611. doi: 10.1371/journal.pone.0201611