.
Condición fitosanitaria: Presente ampliamente distribuida
Grupo de cultivos: Oleaginosas
Especie hospedante: Soja (Glycine max)
Rango de hospedantes: no específico / amplio. S. sclerotiorum es un hongo polífago con un amplio rango de hospedantes y de amplia difusión mundial, siendo el agente causal de podredumbres en diversos cultivos de importancia económica. Se han reportado más de 408 especies de plantas atacadas por S. sclerotiorum, de 278 géneros, en 75 familias. La mayoría de estas especies son dicotiledóneas, aunque también son hospedantes varias plantas monocotiledóneas de importancia agrícola (Boland & Hall, 1994; Bolton et al., 2006; Derbyshire et al., 2022). El grado de susceptibilidad a S. sclerotiorum en las poblaciones de plantas hospedantes a menudo varía considerablemente. Sin embargo, no existe una resistencia completa a S. sclerotiorum en ninguna especie hospedante.
Se han reportado más de 408 especies de plantas atacadas por S. sclerotiorum, de 278 géneros, en 75 familias (Boland & Hall, 1994). Entre las Familias de plantas hospedantes de Scleorotinia sclerotiorum se encuentran:
Actinidiaceae, Aizoaceae, Amaranthaceae, Annonaceae, Apocynaceae, Araliaceae, Aristolochiaceae, Asclepiadaceae, Begoniaceae, Berberidaceae, Boraginaceae, Campanulaceae, Capparidaceae, Caryophyllaceae, Chenopodiaceae, Compositae, Convolvulaceae, Cruciferae, Cucurbitaceae, Dipsacaceae, Euphorbiaceae, Fagaceae, Fumariaceae, Gentianaceae, Geraniaceae, Gesneriaceae, Gramineae, Hippocastanaceae, Iridaceae, Labiatae, Lauraceae, Leguminosae, Liliaceae, Linaceae, Malvaceae, Martyniaceae, Moraceae, Musaceae, Myoporaceae, Myrtaceae, Oleaceae, Onagraceae, Orobanchaceae, Papaveraceae, Passifloraceae, Pinaceae, Plantaginaceae, Polemoniaceae, Polygonaceae, Portulacaceae, Ranunculaceae, Rosaceae, Rutaceae, Saxifragaceae, Scrophulariaceae, Solanaceae, Theaceae, Tilliaceae, Tropaeolaceae, Umbelliferae, Urticaceae, Valerianaceae, Violaceae, Vitaceae.
Epidemiología: monocíclica, subaguda.
Etiología: Hongo. Necrotrófico, con capacidad de supervivencia en el suelo.
Agente causal: Sclerotinia sclerotiorum (Lib.) de Bary (1884)
Taxonomía: Eukaryota > Fungi > Dikarya > Ascomycota > Pezizomycotina > Leotiomycetes > Helotiales > Sclerotiniaceae > Sclerotinia
.
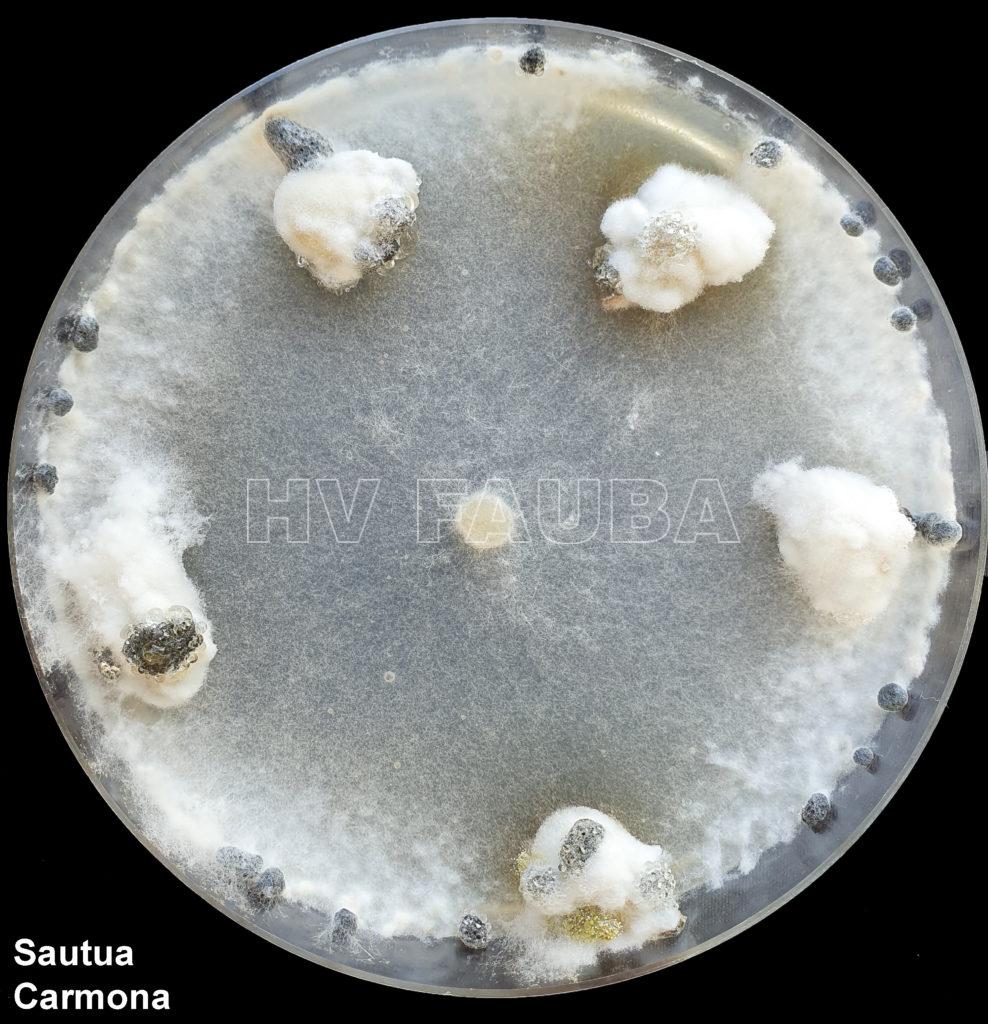
- Sclerotinia sclerotiorum en medio de cultivo especifico (Bromophenol Blue). Autor: Ing. Agr. MSc. Jorge Dominguez
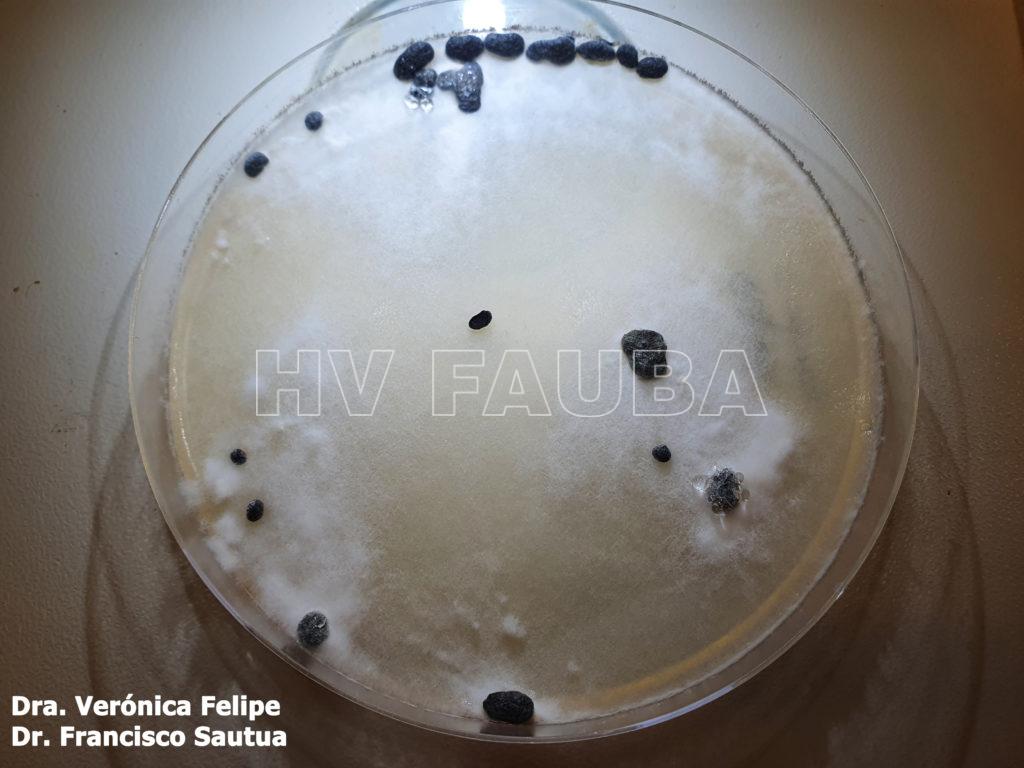
- Autor: Dra. Verónica Felipe, Dr. Francicco Sautua
- Semillas de soja colonizadas por cepa virulenta de Sclerotinia sclerotiorum, con formación de esclerocios luego de 7 días de incubación en agar papa glucosa. Autores: Dr Francisco Sautua, Dr Marcelo Carmona
.
Las especies de Sclerotinia spp. no se reproducen en forma asexual (no forman conidios).
.
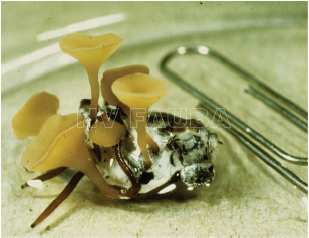
- Apotecios de S. sclerotiorum emergiendo de un esclerocio. Autor: James Venette (publicado en Peltier et al., 2012)
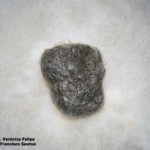
- Autor: Dra. Verónica Felipe, Dr. Francicco Sautua
- Autor: Dra. Verónica Felipe, Dr. Francicco Sautua
- Autor: Dra. Verónica Felipe, Dr. Francicco Sautua
.
.
Síntomas
.
.
.
.
Bibliografía
Albert D, Dumonceaux T, Carisse O, et al. (2022) Combining Desirable Traits for a Good Biocontrol Strategy against Sclerotinia sclerotiorum. Microorganisms 10(6): 1189. doi: 10.3390/microorganisms10061189
Amselem J, Cuomo CA, van Kan JAL, et al. (2011) Genomic analysis of the necrotrophic fungal pathogens Sclerotinia sclerotiorum and Botrytis cinerea. PLoS Genetics 7, e1002230. doi: 10.1371/journal.pgen.1002230
Boland GJ, Hall R (1994) Index of plant hosts of Sclerotinia sclerotiorum. Canadian Journal of Plant Pathology 16: 93-108. doi: 10.1080/07060669409500766
Bolton MD, Thomma BPHJ, Nelson BD (2006) Sclerotinia sclerotiorum (Lib.) de Bary: biology and molecular traits of a cosmopolitan pathogen. Molecular Plant Pathology 7: 1-16. doi: 10.1111/j.1364-3703.2005.00316.x
Botelho SL, Barrocas EN, Machado JC, Martins RS (2015) Detection of Sclerotinia sclerotiorum in soybean seeds by conventional and quantitative PCR techniques. Journal of Seed Science 37(1): 55-62. doi: 10.1590/2317-1545v37n1141460
Cao Y, Zhang X, Song X, et al. (2024) Efficacy and toxic action of the natural product natamycin against Sclerotinia sclerotiorum. Pest Manag Sci. 10.1002/ps.7930
Carpenter KA, Sisson AJ, Kandel YR, et al. (2021) Effects of Mowing, Seeding Rate, and Foliar Fungicide on Soybean Sclerotinia Stem Rot and Yield. Plant Health Progress 22: 129-135. doi: 10.1094/PHP-11-20-0097-RS
Dann E, Diers B, Byrum J. et al. (1998) Effect of treating soybean with 2,6-dichloroisonicotinic acid (INA) and benzothiadiazole (BTH) on seed yields and the level of disease caused by Sclerotinia sclerotiorum in field and greenhouse studies. European Journal of Plant Pathology 104: 271–278. doi: 10.1023/A:1008683316629
Dean R, Van Kan JAL, Pretorius ZA, et al. (2012). The Top 10 fungal pathogens in molecular plant pathology. Molecular Plant Pathology 13: 414–430. doi: 10.1111/j.1364-3703.2011.00783.x
, , (2022) The evolutionary and molecular features of the broad-host-range plant pathogen Sclerotinia sclerotiorum. Molecular Plant Pathology 00: 1– 16. doi: 10.1111/mpp.13221
El-Ashmony RMS, Zaghloul NSS, Milošević M, et al. (2022) The Biogenically Efficient Synthesis of Silver Nanoparticles Using the Fungus Trichoderma harzianum and Their Antifungal Efficacy against Sclerotinia sclerotiorum and Sclerotium rolfsii. Journal of Fungi. 8(6): 597. doi: 10.3390/jof8060597
Gambhir N, Kamvar ZN, Higgins R, et al. (2020) Spontaneous and Fungicide-Induced Genomic Variation in Sclerotinia sclerotiorum. Phytopathology. doi: 10.1094/PHYTO-10-20-0471-FI
Garg H, Li H, Sivasithamparam K, Barbetti MJ (2013) Differentially Expressed Proteins and Associated Histological and Disease Progression Changes in Cotyledon Tissue of a Resistant and Susceptible Genotype of Brassica napus Infected with Sclerotinia sclerotiorum. PLoS ONE 8(6): e65205. doi: 10.1371/journal.pone.0065205
Godoy CL, Koga LJ, Oliveira MCN, et al. (2017) Mycelial growth, pathogenicity, aggressiveness and apothecial development of Sclerotinia sclerotiorum isolates from Brazil and the United States in contrasting temperature regimes. Summa Phytopathologica 43(4): 263-268. doi: 10.1590/0100-5405/2188
Grabicoski EMG, Jaccoud Filho DS, Pileggi M, et al. (2015) Rapid PCR-based assay for Sclerotinia sclerotiorum detection on soybean seeds. Scientia Agricola 72(1): 69-74. doi: 10.1590/0103-9016-2013-0395
Hartman GL, Kull L, Huang YH (1998) Occurrence of Sclerotinia sclerotiorum in Soybean Fields in East-Central Illinois and Enumeration of Inocula in Soybean Seed Lots. Plant Dis. 82(5): 560-564. doi: 10.1094/PDIS.1998.82.5.560
Hossain MM, Sultana F, Rubayet MT, et al. (2025) White Mold: A Global Threat to Crops and Key Strategies for Its Sustainable Management. Microorganisms 13(1): 4. doi: 10.3390/microorganisms13010004
, , , Genome‐wide alternative splicing profiling in the fungal plant pathogen Sclerotinia sclerotiorum during the colonization of diverse host families. Mol Plant Pathology 22: 31– 47. doi: 10.1111/mpp.13006
Kamal MM, Savocchia S, Lindbeck KD, et al. (2016) Biology and biocontrol of Sclerotinia sclerotiorum (Lib.) de Bary in oilseed Brassicas. Australasian Plant Pathol. 45: 1–14. doi: 10.1007/s13313-015-0391-2
Kusch S, Larrouy J, Ibrahim HMM, et al. (2021) Transcriptional response to host chemical cues underpins the expansion of host range in a fungal plant pathogen lineage. ISME J. doi: 10.1038/s41396-021-01058-x
Lehner MS, Pethybridge SJ, Meyer MC, Del Ponte EM (2017) Meta‐analytic modelling of the incidence–yield and incidence–sclerotial production relationships in soybean white mould epidemics. Plant Pathology 66: 460-468. doi: 10.1111/ppa.12590
Lehner MDS, Alves K, Del Ponte EM, Pethybridge SJ (2021) Comparing the Fungicide Sensitivity of Sclerotinia sclerotiorum Using Mycelial Growth and Ascospore Germination Assays. Plant Disease. doi: 10.1094/PDIS-06-21-1234-SC
Peltier AJ, Bradley CA, Chilvers MI, et al. (2012) Biology, Yield loss and Control of Sclerotinia Stem Rot of Soybean. Journal of Integrated Pest Management 3: B1–B7. doi: 10.1603/IPM11033
Macena AMF, Kobori NN, Mascarin GM, et al. (2020) Antagonism of Trichoderma-based biofungicides against Brazilian and North American isolates of Sclerotinia sclerotiorum and growth promotion of soybean. BioControl 65: 235–246. doi: 10.1007/s10526-019-09976-8
McCaghey M, Shao D, Kurcezewski J, et al. (2021) Host-Induced Gene Silencing of a Sclerotinia sclerotiorum oxaloacetate acetylhydrolase Using Bean Pod Mottle Virus as a Vehicle Reduces Disease on Soybean. Front. Plant Sci. 12: 677631. doi: 10.3389/fpls.2021.677631
Mei J, Ding Y, Li Y, et al. (2016) Transcriptomic comparison between Brassica oleracea and rice (Oryza sativa) reveals diverse modulations on cell death in response to Sclerotinia sclerotiorum. Sci Rep 6: 33706. doi: 10.1038/srep33706
Miorini TJJ, Raetano CG, Negrisoli MM, et al. (2021) Determination of the protection period of fungicides used for control of Sclerotinia stem rot in soybean through bioassay and chromatography. Eur J Plant Pathol. doi: 10.1007/s10658-021-02212-z
Moellers TC, Singh A, Zhang J, et al. (2017) Main and epistatic loci studies in soybean for Sclerotinia sclerotiorum resistance reveal multiple modes of resistance in multi-environments. Nature Scientific Reports 7, Article number: 3554. doi: 10.1038/s41598-017-03695-9
Mbengue M, Navaud O, Peyraud R, et al. (2016) Emerging Trends in Molecular Interactions between Plants and the Broad Host Range Fungal Pathogens Botrytis cinerea and Sclerotinia sclerotiorum. Frontiers in Plant Science 7: 422. doi: 10.3389/fpls.2016.00422
O’Sullivan CA, Belt K, Thatcher LF (2021) Tackling Control of a Cosmopolitan Phytopathogen: Sclerotinia. Front. Plant Sci. 12: 707509. doi: 10.3389/fpls.2021.707509
(1979) Sclerotinia sclerotiorum: history, diseases and symptomatology, host range, geographic distribution, and impact. Phytopathology 69: 875–880. doi: 10.1094/Phyto-69-875
Ranjan A, Jayaraman D, Grau C, et al. (2018) The pathogenic development of Sclerotinia sclerotiorum in soybean requires specific host NADPH oxidases. Molecular Plant Pathology 19: 700–714. doi: 10.1111/mpp.12555
(2022) Predicting field diseases caused by Sclerotinia sclerotiorum: A review. Plant Pathology 00: 1– 16. doi: 10.1111/ppa.13643
Saharan GS, Mehta N (2008) Sclerotinia Diseases of Crop Plants: Biology, Ecology and Disease Management. Springer, Dordrecht. 485 p. doi: 10.1007/978-1-4020-8408-9
, , , et al (2024) The schizotrophic lifestyle of Sclerotinia sclerotiorum. Molecular Plant Pathology 25: e13423. doi: 10.1111/mpp.13423
Silva LG, Camargo RC, Mascarin GM, et al. (2022) Dual functionality of Trichoderma: Biocontrol of Sclerotinia sclerotiorum and biostimulant of cotton plants. Front. Plant Sci. 13: 983127. doi: 10.3389/fpls.2022.983127
Singh M, Avtar R, Lakra N, et al. (2021) Genetic and Proteomic Basis of Sclerotinia Stem Rot Resistance in Indian Mustard [Brassica juncea (L.) Czern & Coss.]. Genes 12(11): 1784. doi: 10.3390/genes12111784
Smolińska U, Kowalska B (2018) Biological control of the soil-borne fungal pathogen Sclerotinia sclerotiorum – a review. J Plant Pathol 100: 1–12. doi: 10.1007/s42161-018-0023-0
Willbur J, McCaghey M, Kabbage M, et al. (2018) An overview of the Sclerotinia sclerotiorum pathosystem in soybean: impact, fungal biology, and current management strategies. Tropical Plant Pathology pp 1–9. doi: 10.1007/s40858-018-0250-0
Willbur JF, Fall ML, Bloomingdale C, et al. (2018) Weather-Based Models for Assessing the Risk of Sclerotinia sclerotiorum Apothecial Presence in Soybean (Glycine max) Fields. Plant Disease 102(1): 73-84. doi: 10.1094/PDIS-04-17-0504-RE
Taylor A, Coventry E, Handy C, et al. (2018) Inoculum potential of Sclerotinia sclerotiorum sclerotia depends on isolate and host plant. Plant Pathology (in press). doi: 10.1111/ppa.12843
Wei W, Xu L, Peng H, et al. (2022) A fungal extracellular effector inactivates plant polygalacturonase-inhibiting protein. Nat Commun 13: 2213. doi: 10.1038/s41467-022-29788-2
Xu L, Li G, Jiang D, Chen W (2018) Sclerotinia sclerotiorum: An Evaluation of Virulence Theories. Annual Review of Phytopathology 56: 311-338. doi: 10.1146/annurev-phyto-080417-050052
Yang XB, Workneh F, Lundeen P (1998) First Report of Sclerotium Production by Sclerotinia sclerotiorum in Soil on Infected Soybean Seeds. Plant Dis. 82(2): 264. doi: 10.1094/PDIS.1998.82.2.264B
Yu S-F, Wang C-L, Hu Y-F, et al. (2022) Biocontrol of Three Severe Diseases in Soybean. Agriculture 12(9): 1391. doi: 10.3390/agriculture12091391
Zeng W, Wang D, Kirk W, Hao J (2012) Use of Coniothyrium minitans and other microorganisms for reducing Sclerotinia sclerotiorum. Biol Control 60: 225–232. doi: 10.1016/j.biocontrol.2011.10.009
Zhang M, Liu Y, Li Z (2021) The bZIP transcription factor GmbZIP15 facilitates resistance against Sclerotinia sclerotiorum and Phytophthora sojae infection in soybean. iScience 24(6): 102642. doi: 10.1016/j.isci.2021.102642
Zhao X, Han Y, Li Y, et al. (2015) Loci and candidate gene identification for resistance to Sclerotinia sclerotiorum in soybean (Glycine max L. Merr.) via association and linkage maps. The Plant Journal 82: 245–255. doi: 10.1111/tpj.12810