.
Condición fitosanitaria: Presente
Grupo de cultivos: Cereales
Especie hospedante: Trigo (Triticum aestivum)
Rango de hospedantes: Ptr tiene un amplio rango de hospedantes que incluye varios hospedantes herbáceos cultivados, como T. aestivum (trigo harinero), T. turgidum (trigo duro), Hordeum vulgare (cebada) y Secale cereale (centeno), pero también muchas otras especies de gramíneas (malezas) (Wegulo, 2011).
Etiología: Hongo. Necrotrófico
Epidemiología: policíclica, subaguda.
Agente causal: Drechslera tritici-repentis (anamorfo), Pyrenophora tritici-repentis (Died.) Drechsler 1923 (teleomorfo)
Taxonomía: Eukaryota > Fungi > Dikarya > Ascomycota > Pezizomycotina > Dothideomycetes > Pleosporomycetidae > Pleosporales > Pleosporaceae > Pyrenophora
.
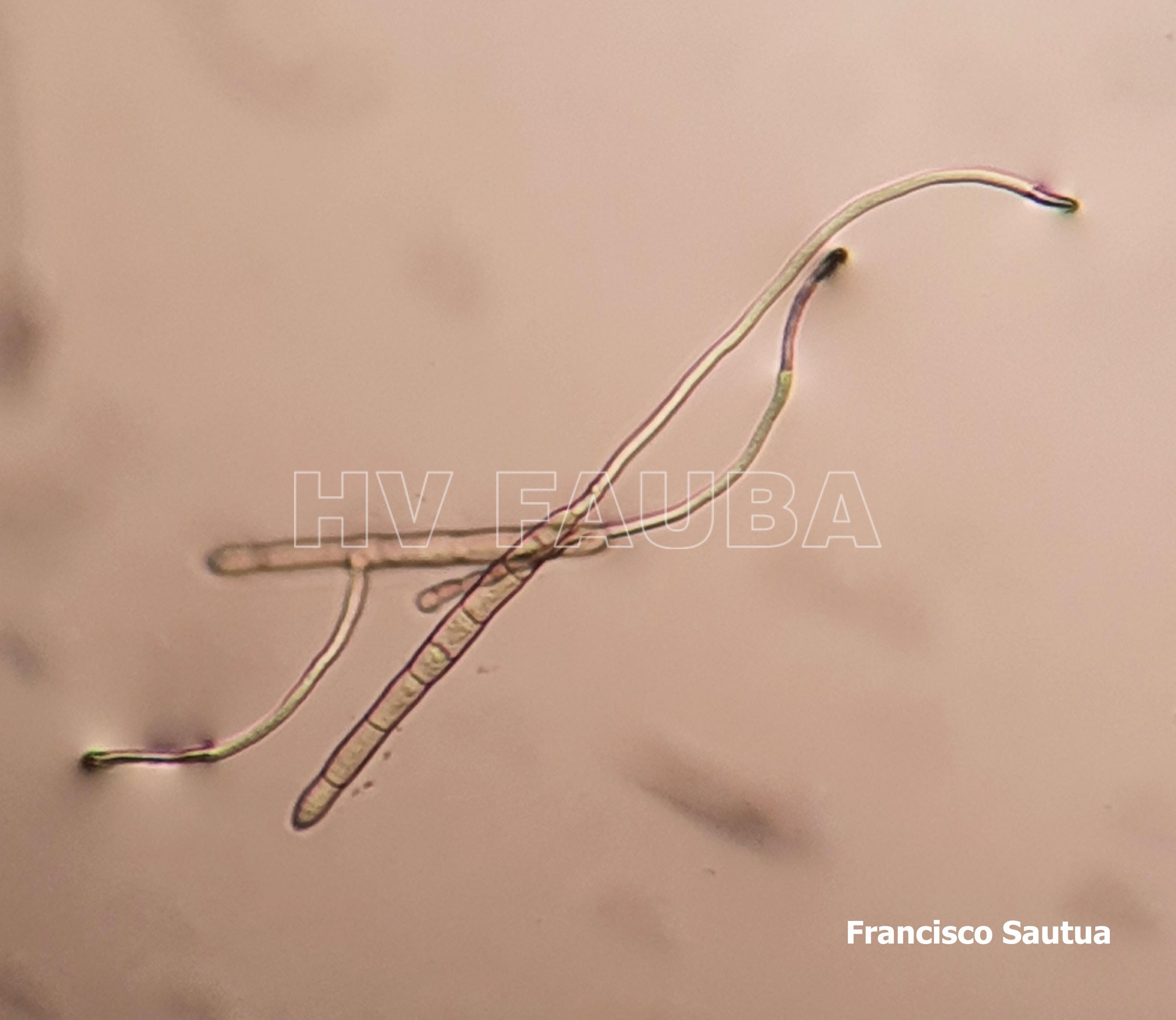
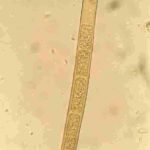
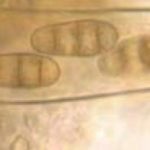
- Conidios de Drechslera tritici-repentis germinados en agar agua. Autor: Francisco Sautua
- Conidios de Drechslera tritici-repentis, con la característica «punta de lanza» o «cabeza de serpiente».
.
.
Antecedentes
Pyrenophora tritici-repentis fue descrito por primera vez como un patógeno del trigo en Japón en la década de 1920 (Hafez et al., 2022).
.
Síntomas
Mancha parda o marrón, con un halo amarillento característico alrededor de la misma. Aparecen principalmente en hojas, de todas maneras, los síntomas más comunes se observan en hojas y vainas. También puede infectar espiguillas (See et al., 2020). Esta enfermedad es comúnmente llamada a) Mancha amarilla debido a los halos amarillentos pronunciados alrededor de las lesiones, b) mancha bronceada porque la región central presenta una coloración parda y c) Helminthosporiosis porque el agente causal perteneció en el pasado al género Helminthosporium.
La enfermedad no manifiesta signo a campo (los conidios no se observan a ojo desnudo).
.
- Autor: Dr. Marcelo Carmona
- Mancha amarilla en Klein100 años, Navarro, Bs As. Autores: Silvana Di Nubila, Marcelo Carmona
- Mancha amarilla en Klein100 años, Navarro, Bs As. Autores: Silvana Di Nubila, Marcelo Carmona
.
.
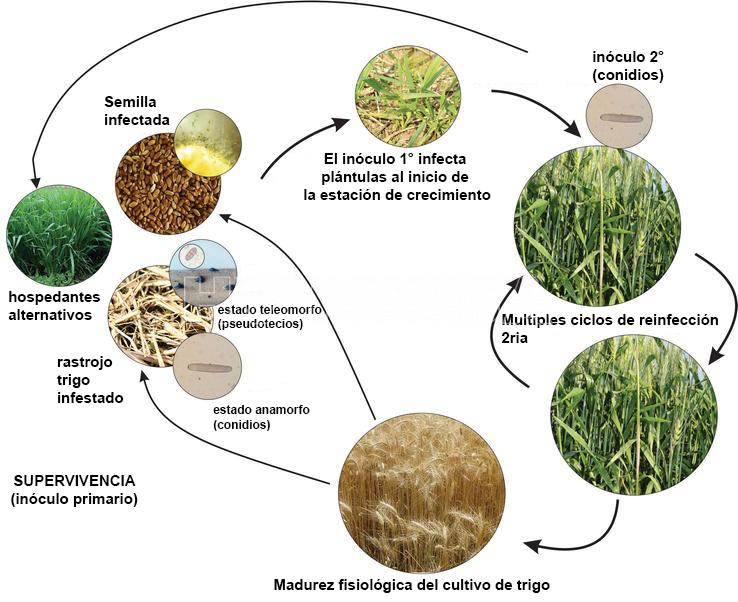
Ciclo de la enfermedad y Epidemiología
P. tritici-repentis sobrevive a los períodos de ausencia de hospedante agronómico como pseudotecios en los residuos o restos culturales (rastrojos) del hospedante principalmente. Los pseudotecios se desarrollan y maduran en el rastrojo de trigo durante el otoño e invierno. Esta se considera tradicionalmente como la principal fuente de inóculo primario. Otras fuentes de inóculo primario incluyen semillas infectadas, trigo voluntario y otras especies de gramíneas (hospedantes alternativos o secundarios). En estos casos, el inóculo es principalmente en forma de conidios. En la primavera, las ascosporas se descargan desde los pseudotecios, se dispersan por el viento, y causan infecciones primarias en las hojas. La descarga de ascosporas se ve favorecida por la lluvia, la alta humedad relativa y las temperaturas superiores a 10°C. Los conidios producidos en las lesiones en maduración de las hojas sirven como inóculo secundario. Sin embargo, los conidios también se pueden producir en rastrojos y servir como inóculo primario (Krupinsky, 1992). Las conidios se producen en mayor número y, debido a su gran tamaño y peso, su dispersión es solo a distancias cortas por el viento. Por lo tanto, son epidemiológicamente más importantes que las ascosporas. Durante y después de la cosecha de trigo, el hongo crece como micelio desde la lámina de la hoja infectada hacia abajo de la vaina de la hoja y hacia el tallo donde más tarde formará pseudotecios. Los estudios indican que las ascosporas pueden servir para dispersar P. tritici-repentis a cortas distancias (Schilder y Bergstrom, 1992).
El clima no es limitante para la ocurrencia de la enfermedad. Se desarrolla en un rango de temperatura entre 18 y 28ºC, y requiere para su infección de alrededor de 30 horas de mojado foliar. El hongo permanece en el rastrojo y en la semilla (See et al., 2020), siendo éstas las principales fuentes de inóculo primario. El patógeno forma ascosporas en pseudotecios y conidios en conidióforos libres. En Argentina y en el resto del Cono Sur, en los últimos años, se ha registrado un aumento de la mancha amarilla, principalmente sobre cultivos de trigo bajo monocultivo y siembra directa. La diseminación a grandes distancias es por semillas infectadas. El hongo penetra por apresorios. El patrón de distribución en el lote es generalizada y uniforme.
.
- Ciclo de la mancha amarilla del trigo, causada por Pyrenophora tritici-repentisAutor: Roman Ramos et al., 2023
.
.
Condiciones predisponentes:
A 20°C, la mayoría de las infecciones ocurren entre 6 y 24 horas en presencia de humedad libre (mojado foliar). En general, se requiere un período húmedo de 6 a 48 horas para que ocurra la infección. La duración del período húmedo requerido para la infección depende de la temperatura predominante. Es más corto en condiciones cálidas y más largo en climas fríos. Los síntomas aparecen entre cinco y siete días después de la infección (período de incubación).
* La siembra de semillas infectadas introduce la enfermedad en campos nuevos o bajo rotación.
* El monocultivo asegura la presencia indefinida del patógeno en el cultivo. Resulta más grave cuando se trata de siembra directa.
* Temperatura 18-28 ºC, humedad relativa elevada en el período de cultivo (de 6 a 48 horas) y para el caso de las infecciones secundarias además se requiere viento y lluvia..
.
Daños
De las enfermedades del trigo, se puede considerar que la mancha amarilla es la principal. Las pérdidas estimadas para la Argentina varían del 20 al 25 %. Afecta principalmente el peso de granos. En ataques intensos puede provocar la pérdida de hojas y un menor número de granos/espiga.
.
Manejo de la enfermedad
Las medidas preferenciales (económicas y antes de la siembra) son: tratamiento eficiente de semillas y rotación de cultivos. Ambas deben ser llevadas a cabo complementariamente.
* Tratamiento de semillas: Los fungicidas actualmente usados en Argentina para controlar los carbones no resultan eficientes para controlar al género Drechslera. Se puede consultar bibliografía en el trabajo » Control químico de semillas de cereales de invierno » , Carmona, M & Erlei Melo Reis Actas de Conferencias del 5to Congreso Nacional de AAPRESID, pp. 70-77, 1998.
* Rotación de cultivos: El trigo debería ser cultivado en el mismo lote, sólo después de la completa mineralización de los restos culturales. Rotaciones con colza, lino y avena resultan exitosas para el manejo de la enfermedad.
* Aplicación de fungicidas foliares: Esta práctica es recomendada en cultivos donde la enfermedad ya está presente alcanzando un nivel de daño que justifique el control químico. Entre los fungicidas recomendados los más eficientes son los triazoles sistémicos.
* Eliminación de plantas guachas: Tales plantas garantizan la supervivencia del patógeno en el verano‑otoño, actuando como puentes «verdes» y comprometiendo el período de rotación necesario.
* Resistencia varietal: La mayoría de los cultivares de trigo son susceptibles en diversos grados a la mancha amarilla, aunque existen algunos genotipos de trigo que tienen mejor comportamiento. Se han reportado fuentes de resistencia poligénica (QTLs) o cuantitativa a diferentes razas de Ptr (Singh et al., 2008a, 2008b; Gurung et al., 2011, 2014; Patel et al., 2013; Shankar et al., 2017; Dinglasan et al., 2018, 2021; Liu et al., 2020).
.
.
- Síntomas de mancha amarilla, lesiones necróticas con halo clorótico en plántulas de trigo inoculadas. Autor: Dylan Mangel, KSU.
- Esporulación (conidios sobre conidióforos libres) sobre lesión de mancha amarilla. Autor: Dylan Mangel, KSU.
- Esporulación in vitro de Pyrenophora tritici-repentis en medio de cultivo V8. Autores: Francisco Sautua, Marcelo Carmona.
- Esporulación in vitro de Pyrenophora tritici-repentis en medio de cultivo V8. Autores: Francisco Sautua, Marcelo Carmona.
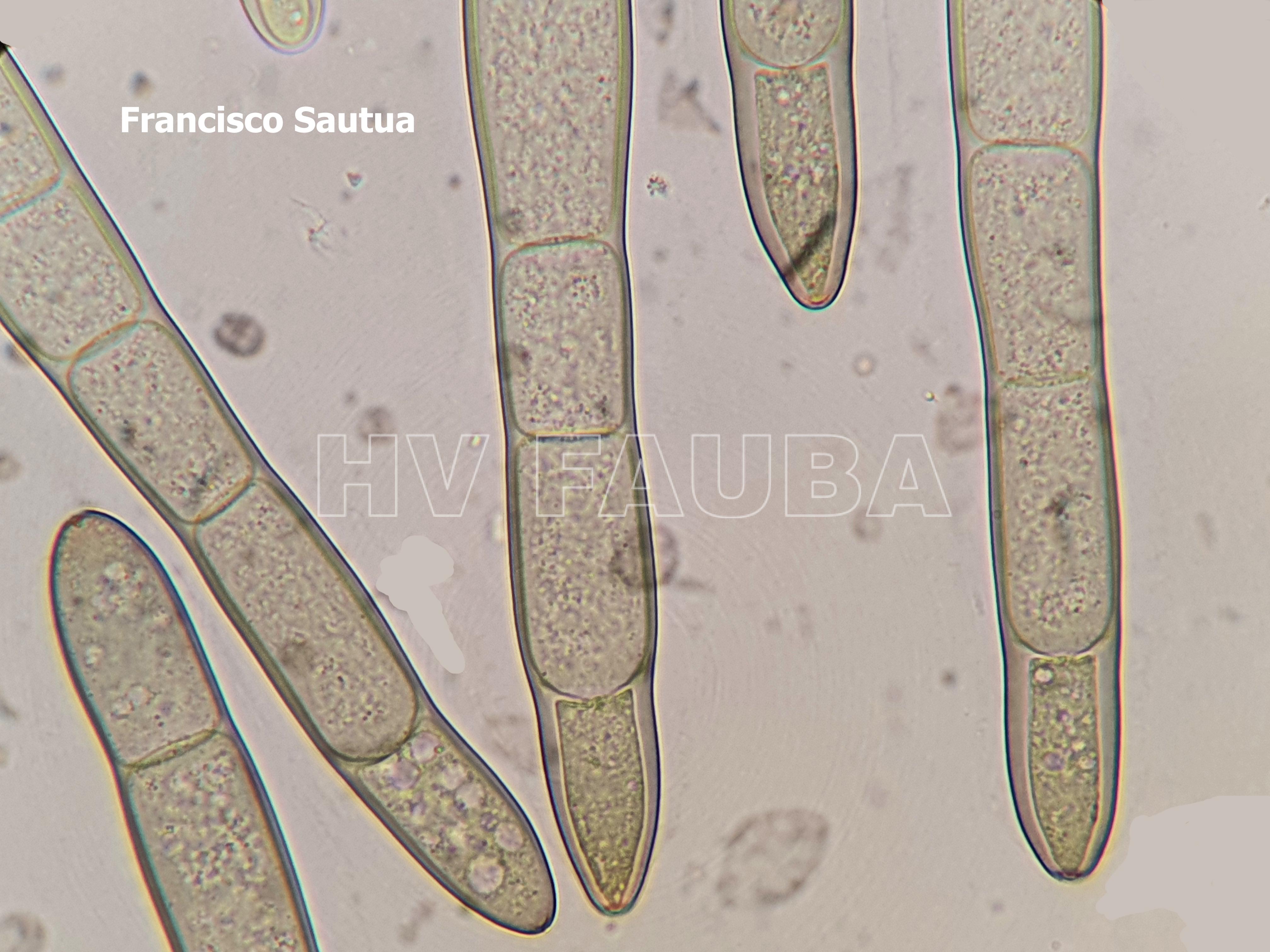
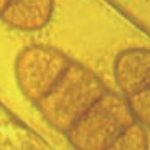
- Hifa y conidio de Pyrenophora tritici-repentis. Autores: Francisco Sautua, Marcelo Carmona.
- Conidio de Drechslera tritici-repentis. Autor: Dr. Marcelo Carmona
- Conidio de Pyrenophora tritici-repentis germinando in vitro luego de 8 hs desde la inducción de la germinación. Autores: Francisco Sautua, Marcelo Carmona.
- Pseudotecios de Pyrenophora tritici-repentis sobre rastrojo de trigo. Autor: Ing. Agr. MSc. Cecilia Smirnoff
- Pseudotecios de Pyrenophora tritici-repentis sobre rastrojo de trigo. Autor: Ing. Agr. MSc. Cecilia Smirnoff
- Ascos con 8 ascosporas de Pyrenophora tritici-repentis. Autor: Benslimane H.
- Ascos con 8 ascosporas de Pyrenophora tritici-repentis. Autor: Benslimane H.
- Autor: Dirceu Gassen
- Autor: Dirceu Gassen
- Autor: Dirceu Gassen
- Autor: Dirceu Gassen
- Autor: Dirceu Gassen
- Autor: Dirceu Gassen
- Autor: Dirceu Gassen
- Autor: Dirceu Gassen
- Autor: Dirceu Gassen
- Autor: Dirceu Gassen
.
.
.
Videos
Tan Spot of Wheat Preview Clip
.
.
Bibliografía
Abdullah S, Sehgal SK, Ali S, et al. (2017) Characterization of Pyrenophora tritici-repentis (Tan Spot of Wheat) Races in Baltic States and Romania. The plant pathology journal, 33(2), 133-139. doi: 10.5423/PPJ.OA.10.2016.0214
Aboukhaddour R, Cloutier S, Ballance GM, Lamari L (2009) Genome characterization of Pyrenophora tritici-repentis isolates reveals high plasticity and independent chromosomal location of ToxA and ToxB. Mol Plant Pathol. 10(2): 201-212. doi: 10.1111/j.1364-3703.2008.00520.x
Aboukhaddour R, Cloutier S, Lamari L, Strelkov S (2011) Simple sequence repeats and diversity of globally distributed populations of Pyrenophora tritici-repentis. Canadian Journal of Plant Pathology 33: 389-399. doi: 10.1080/07060661.2011.590821
Aboukhaddour R, Kim YM, Strelkov SE (2012) RNA‐mediated gene silencing of ToxB in Pyrenophora tritici‐repentis. Molecular Plant Pathology 13: 318-326. doi: 10.1111/j.1364-3703.2011.00748.x
Aboukhaddour R, Turkington TK, Strelkov SE (2013) Race structure of Pyrenophora triciti-repentis (tan spot of wheat) in Alberta, Canada. Canadian Journal of Plant Pathology 35: 256-268.
Aboukhaddour R, Hafez M, Strelkov SE, Fernandez MR (2021) Tan spot disease under the lenses of plant pathologists. In Achieving durable disease resistance in cereals (pp. 589-621). Burleigh Dodds Science Publishing.
Aboukhaddour R, Turkington TK, Strelkov SE (2013) Race structure of Pyrenophora triciti-repentis (tan spot of wheat) in Alberta, Canada. Canadian journal of plant pathology 35(2): 256-268. doi: 10.1080/07060661.2013.782470
Aboukhaddour R, Hafez Abdel-Fattah M, McDonald M, et al. (2023) A revised nomenclature for ToxA haplotypes across multiple fungal species. Phytopathology. doi: 10.1094/PHYTO-01-23-0017-SC
Adhikari TB, Bai J, Meinhardt SW, et al. (2009) Tsn1-mediated host responses to ToxA from Pyrenophora tritici-repentis. Mol Plant Microbe Interact. 22(9): 1056-68. doi: 10.1094/MPMI-22-9-1056
Ali S, Francl LJ (2001) Recovery of Pyrenophora tritici-repentis from Barley and Reaction of 12 Cultivars to Five Races and Two Host-Specific Toxins. Plant Disease 85(6): 580‐584. doi: 10.1094/PDIS.2001.85.6.580
Ali S, Francl LJ (2003) Population Race Structure of Pyrenophora tritici-repentis Prevalent on Wheat and Noncereal Grasses in the Great Plains. Plant Dis. 87(4):418-422. doi: 10.1094/PDIS.2003.87.4.418
Ali S, Gurung S, Adhikari TB (2010) Identification and Characterization of Novel Isolates of Pyrenophora tritici-repentis from Arkansas. Plant Disease 94(2): 229-235. doi: 10.1094/PDIS-94-2-0229
Ali S, Francl LJ, De Wolf ED (1999) First Report of Pyrenophora tritici-repentis Race 5 from North America. Plant Disease 83(6): 591. doi: 10.1094/PDIS-03-16-0295-PDN
Ali S, Langham MA (2015) Reaction of Five Non-cereal Grasses to Five Races and Two Host Selective Toxins of Pyrenophora tritici-repentis. The Plant Pathology Journal 31(3): 245-51. doi: 10.5423/PPJ.OA.03.2015.0028
Amaike S, Ozga JA, Basu U, Strelkov SE (2008) Quantification of ToxB gene expression and formation of appressoria by isolates of Pyrenophora tritici‐repentis differing in pathogenicity. Plant Pathology 57: 623-633. doi: 10.1111/j.1365-3059.2007.01821.x
Ameen G, Kariyawasam G, Shi G, et al. (2017) Molecular manipulation of the mating-type system and development of a new approach for characterizing pathogen virulence in Pyrenophora tritici-repentis. Fungal Genetics and Biology 109: 16-25. doi: 10.1016/j.fgb.2017.10.004
Andersen EJ, Ali S, Nepal MP (2019) Transcriptomic changes in wheat during tan spot (Pyrenophora tritici-repentis) disease. BMC Res Notes 12: 471. doi: 10.1186/s13104-019-4517-4
Andersen EJ, Nepal MP, Ali S (2021) Necrotrophic Fungus Pyrenophora tritici-repentis Triggers Expression of Multiple Resistance Components in Resistant and Susceptible Wheat Cultivars. Plant Pathol J. 37(2): 99-114. doi: 10.5423/PPJ.OA.06.2020.0109
Andersson B, Djurle A, Ørum JE, et al. (2022) Comparison of models for leaf blotch disease management in wheat based on historical yield and weather data in the Nordic-Baltic region. Agron. Sustain. Dev. 42: 42. doi: 10.1007/s13593-022-00767-7
Andrie RM, Schoch CL, Hedges R, et al. (2008) Homologs of ToxB, a host-selective toxin gene from Pyrenophora tritici-repentis, are present in the genome of sister-species Pyrenophora bromi and other members of the Ascomycota. Fungal Genetics and Biology 45(3): 363-377. doi: 10.1016/j.fgb.2007.10.014
Annone JG (1985) Presencia de la “mancha tostada” del trigo (Helminthosporium tritici-repentis). Carpeta de Produccion Vegetal. TRIGO. TOMO VII. Inf. No. 88. EEA INTA, Pergamino, Buenos Aires.
Annone JG (1990) Importancia y distribucion de las septoriosis en la Argentina. In: Kohli, M.M. & van Beuningen, L.T. (Eds.) Conferencia Regional sobre la Septoriosis del Trigo. Montevideo. México: CIMMYT, pp. 9–14. Link
Annone J (1998) Tan spot of wheat in Argentina: Importance and disease management strategies. In: Duveiller E, Dubin H, Reeves J, McNab A, editors; Mexico, D.F. CIMMYT. pp. 339–345.
Asaturova A, Zhevnova N, Tomashevich N, et al. (2022) Efficacy of New Local Bacterial Agents against Pyrenophora tritici-repentis in Kuban Region, Russia. Agronomy 12(2): 373. doi: 10.3390/agronomy12020373
Barrus MF (1942) A disease of wheat newly recorded for this country – yellow spot disease of wheat in New York State. Plant Dis. Reporter 26: 246.
Benslimane H (2014) Simple Method to Produce in vitro Pyrenophora tritici-repentis Teleomorph. Plant Pathol J. 30(4): 437-9. doi: 10.5423/PPJ.NT.06.2014.0049
Benslimane H, Aouali S, Khalfi A, et al. (2017) In Vitro Morphological Characteristics of Pyrenophora tritici-repentis Isolates from Several Algerian Agro-Ecological Zones. Plant Pathol J. 33(2): 109-117. doi: 10.5423/PPJ.OA.09.2015.0189
Bertagnolli VV, Ferreira JR, Liu Z, et al. (2019) Phenotypical and genotypical characterization of Pyrenophora tritici-repentis races in Brazil. Eur J Plant Pathol 154: 995–1007. doi: 10.1007/s10658-019-01720-3
Betts MF, Manning VA, Cardwell KB, et al. (2011) The importance of the N-terminus for activity of Ptr ToxB, a chlorosis-inducing host-selective toxin produced by Pyrenophora tritici-repentis. Physiological and molecular plant pathology 75(4): 138-145. doi: 10.1016/j.pmpp.2011.03.002
Bugingo C, Yabwalo D, Shires M, etc. (2025) Microbial Biopesticides’ Efficacy in the Management of Tan spot in Wheat. Plant Disease. doi: 10.1094/PDIS-03-25-0571-SC
Carmona MA, Ferrazini M, Barreto DE (2006) Tan Spot of Wheat Caused by Drechslera tritici-repentis: Detection, Transmission, and Control in Wheat Seed. Cereal Research Communications 34: 1043–1049. doi: 10.1556/CRC.34.2006.2-3.236
Chen H, Xue LH, White JF, et al. (2021) Identification and Characterization of Pyrenophora Species Causing Leaf Spot on Oat (Avena sativa) in Western China. Plant Pathology. doi: 10.1111/ppa.13491
Ciuffetti LM, Tuori RP, Gaventa JM (1997) A single gene encodes a selective toxin causal to the development of tan spot of wheat. The Plant Cell 9(2): 135-144. doi: 10.1105/tpc.9.2.135
Ciuffetti LM, Francl LJ, Ballance GM, et al. (1998) Standardization of toxin nomenclature in the Pyrenophora tritici-repentis/wheat interaction. Canadian Journal of Plant Pathology 20(4): 421-424. doi: 10.1080/07060669809500415
Ciuffetti LM, Tuori RP (1999) Advances in the characterization of the Pyrenophora tritici-repentis–wheat interaction. Phytopathology 89: 444-449. doi: 10.1094/PHYTO.1999.89.6.444
Ciuffetti LM, Manning VA, Pandelova I, et al. (2010) Host-selective toxins, Ptr ToxA and Ptr ToxB, as necrotrophic effectors in the Pyrenophora tritici-repentis–wheat interaction. New Phytologist 187: 911–919. doi: 10.1111/j.1469-8137.2010.03362.x
Ciuffetti L.M. et al. (2014) Pyrenophora tritici-repentis: A Plant Pathogenic Fungus with Global Impact. In: Dean R, Lichens-Park A, Kole C (eds) Genomics of Plant-Associated Fungi: Monocot Pathogens. Springer, Berlin, Heidelberg. doi: 10.1007/978-3-662-44053-7_1
Corsi B, Percival-Alwyn L, Downie RC, et al. (2020) Genetic analysis of wheat sensitivity to the ToxB fungal effector from Pyrenophora tritici-repentis, the causal agent of tan spot. Theor Appl Genet 133: 935–950. doi: 10.1007/s00122-019-03517-8
da Luz WC, Bergstrom GC (1986) Effect of temperature on tan spot development in spring wheat cultivars differing in resistance. Canadian Journal of Plant Pathology 8: 451-454. doi: 10.1080/07060668609501786
(1998) Vistas of tan spot research. Canadian Journal of Plant Pathology 20: 349-370. doi: 10.1080/07060669809500404
Dinglasan EG, Godwin ID, Phan HTT, et al. (2018) Vavilov wheat accessions provide useful sources of resistance to tan spot (syn. yellow spot) of wheat. Plant Pathology 67: 1076-1087. doi: 10.1111/ppa.12822
Dinglasan EG, Peressini T, Marathamuthu KA, et al. (2021) Genetic characterization of adult-plant resistance to tan spot (syn, yellow spot) in wheat. Theor Appl Genet. doi: 10.1007/s00122-021-03861-8
Dorneles KR, Pazdiora PC, Hoffmann JF, et al. (2018) Wheat leaf resistance to Pyrenophora tritici‐repentis induced by silicon activation of phenylpropanoid metabolism. Plant Pathology 67: 1713-1724. doi: 10.1111/ppa.12876
Duczek LJ, Sutherland KA, Reed SL, et al. (1999) Survival of leaf spot pathogens on crop residues of wheat and barley in Saskatchewan. Canadian Journal of Plant Pathology 21: 165-173. doi: 10.1080/07060669909501208
Duff AD (1954) A new disease of wheat in Kenya caused by a species of Pyrenophora. East Afr. Agric. J. 19: 225–228.
Dushnicky LG, Ballance GM, Sumner MJ, MacGregor AW (1996) Penetration and infection of susceptible and resistant wheat cultivars by a necrosis toxin-producing isolate of Pyrenophora tritici-repentis. Can. J. Plant Pathol. 18: 392-402. doi: 10.1080/07060669609500594
Dutilloy E, Oni FE, Esmaeel Q, et al. (2022) Plant Beneficial Bacteria as Bioprotectants against Wheat and Barley Diseases. Journal of Fungi. 8(6): 632. doi: 10.3390/jof8060632
Effertz RJ, Anderson JA, Francl LJ (2001) Restriction fragment length polymorphism mapping of resistance to two races of Pyrenophora tritici-repentis in adult and seedling wheat. Phytopathology 91: 572-578. doi: 10.1094/PHYTO.2001.91.6.572
Effertz RJ, Meinhardt SW, Anderson JA, et al. (2002) Identification of a chlorosis-inducing toxin from Pyrenophora tritici-repentis and the chromosomal location of an insensitivity locus in wheat. Phytopathology 92: 527-533. doi: 10.1094/PHYTO.2002.92.5.527
Ellwood SR, Syme RA, Moffat CS, Oliver RP (2012) Evolution of three Pyrenophora cereal pathogens: recent divergence, speciation and evolution of non-coding DNA. Fungal Genetics and Biology 49(10):825-829. doi: 10.1016/j.fgb.2012.07.003
Ferreira LC, Santana FM, Scagliusi SMM, et al. (2024) Induced responses to the wheat pathogen: Tan Spot—(Pyrenophora tritici-repentis) in wheat (Triticum aestivum) focus on changes in defence associated and sugar metabolism. Metabolomics 20: 19. doi: 10.1007/s11306-023-02084-w
Ferreira LC, Santana FM, Scagliusi SMM, et al. (2025) Omic characterisation of multi-component defences against the necrotrophic pathogen Pyrenophora tritici-repentis in wheat. Plant Biol J. doi: 10.1111/plb.13746
Faris JD, Liu Z, Xu SS (2013) Genetics of tan spot resistance in wheat. Theor. Appl. Genet. 126: 2197-2217. doi: 10.1007/s00122-013-2157-y
Figueroa M, Manning VA, Pandelova I, Ciuffetti LM (2015) Persistence of the host-selective toxin Ptr ToxB in the apoplast. Molecular Plant-Microbe Interactions 28(10): 1082-1090. doi: 10.1094/MPMI-05-15-0097-R
Figueroa M, Hammond‐Kosack KE, Solomon PS (2018) A review of wheat diseases—a field perspective. Molecular Plant Pathology 19: 1523-1536. doi: 10.1111/mpp.12618
Fleitas MC, Schierenbeck M, Gerard GS (2018) Breadmaking quality and yield response to the green leaf area duration caused by fluxapyroxad under three nitrogen rates in wheat affected with tan spot. Crop Protection 106: 201-209. doi: 10.1016/j.cropro.2018.01.004
Friesen TL, Ali S, Stack RW, et al. (2003) Rapid and efficient production of the Pyrenophora tritici-repentis teleomorph. Canadian Journal of Botany 81(8): 890-895. doi: 10.1139/b03-082
Friesen TL, Stukenbrock EH, Liu Z, et al. (2006) Emergence of a new disease as a result of interspecific virulence gene transfer. Nature Genetics 38: 953–956. doi: 10.1038/ng1839
Friesen TL, Faris JD, Solomon PS, Oliver RP (2008) Host-specific toxins: effectors of necrotrophic pathogenicity. Cell Microbiol. 10(7): 1421-8. doi: 10.1111/j.1462-5822.2008.01153.x
Friesen TL, Faris JD (2021) Characterization of Effector-Target Interactions in Necrotrophic Pathosystems Reveals Trends and Variation in Host Manipulation. Annu Rev Phytopathol. 59: 77-98. doi: 10.1146/annurev-phyto-120320-012807
Gamba FM, Strelkov SE, Lamari L (2012) Virulence of Pyrenophora tritici-repentis in the Southern Cone Region of South America. Can. J. Plant Pathol. 34: 545-550. doi: 10.1080/07060661.2012.695750
Gaurav K, Arora S, Silva P, et al. (2021) Population genomic analysis of Aegilops tauschii identifies targets for bread wheat improvement. Nat Biotechnol. doi: 10.1038/s41587-021-01058-4
Gourlie R, McDonald M, Hafez M, et al. (2022) The pangenome of the wheat pathogen Pyrenophora tritici-repentis reveals novel transposons associated with necrotrophic effectors ToxA and ToxB. BMC Biol 20: 239. doi: 10.1186/s12915-022-01433-w
Guo J, Shi G, Liu Z (2018) Characterizing Virulence of the Pyrenophora tritici-repentis Isolates Lacking Both ToxA and ToxB Genes. Pathogens 7(3): 74. doi: 10.3390/pathogens7030074
Guo J, Shi G, Kalil A, et al. (2020) Pyrenophora tritici-repentis Race 4 Isolates Cause Disease on Tetraploid Wheat. Phytopathology 110(11): 1781-1790. doi: 10.1094/PHYTO-05-20-0179-R
Gupta PK, Vasistha NK, Singh PK, et al. (2021) Sensitivity genes in wheat and corresponding effector genes in necrotrophs exhibiting inverse gene-for-gene relationship. PREPRINT (Version 1) available at Research Square. doi: 10.21203/rs.3.rs-223024/v1
Gupta PK, Vasistha NK, Singh S, Joshi AK (2023) Genetics and breeding for resistance against four leaf spot diseases in wheat (Triticum aestivum L.). Front Plant Sci. doi: 10.3389/fpls.2023.1023824
Gurung S, Mamidi S, Bonman JM, et al. (2011) Identification of novel genomic regions associated with resistance to Pyrenophora tritici-repentis races 1 and 5 in spring wheat landraces using association analysis. Theoretical and Applied Genetics 123: 1029. doi: 10.1007/s00122-011-1645-1
Gurung S, Mamidi S, Bonman JM, et al. (2014) Genome-Wide Association Study Reveals Novel Quantitative Trait Loci Associated with Resistance to Multiple Leaf Spot Diseases of Spring Wheat. PLoS ONE 9(9): e108179. doi: 10.1371/journal.pone.0108179
Hafez M, Despins T, Nakajima K, Aboukhaddour R (2022) Identification of a novel ToxA haplotype of Pyrenophora tritici-repentis from Japan. Phytopathology. doi: 10.1094/PHYTO-01-22-0001-SC
Hafez M, Gourlie R, McDonald M, et al. (2023) Evolution of the ToxB gene in Pyrenophora tritici-repentis and related species. Mol Plant Microbe Interact. doi: 10.1094/MPMI-08-23-0114-FI
He X, Gahtyari NC, Roy C, et al. (2022) Globally Important Non-rust Diseases of Wheat. In: Reynolds MP, Braun HJ (eds) Wheat Improvement. Springer, Cham. doi: 10.1007/978-3-030-90673-3_9
Hjelkrem AGR, Ficke A, Abrahamsen U, et al. (2021) Prediction of leaf Bloch disease risk in Norwegian spring wheat based on weather factors and host phenology. European Journal of Plant Pathology.doi: 10.1007/s10658-021-02235-6
Hosford RM, Hammond JJ (1987) Interaction of wet period and temperature on Pyrenophora tritici-repentis infection and development in wheats of differing resistance. doi: 10.1094/Phyto-77-1021
Iqbal M, Semagn K, Jarquin D, et al. (2022) Identification of Disease Resistance Parents and Genome-Wide Association Mapping of Resistance in Spring Wheat. Plants 11(21): 2905. doi: 10.3390/plants11212905
Jacques S, Lenzo L, Stevens K, et al. (2021) An optimized sporulation method for the wheat fungal pathogen Pyrenophora tritici-repentis. Plant Methods 17: 52. doi: 10.1186/s13007-021-00751-4
Johnson AJ (1942) Helminthosporium tritici-vulgaris on wheat in Maryland. Plant Dis. Reporter 26: 246–247.
Jørgensen LN, Olsen LV (2007) Control of tan spot (Drechslera tritici-repentis) using cultivar resistance, tillage methods and fungicides. Crop Protection 26: 1606-1616. doi: 10.1016/j.cropro.2007.01.009
Kader KA, Hunger RM, Sreedharan A, et al. (2021) Races, disease symptoms and genetic variability in Pyrenophora tritici-repentis isolates from Oklahoma that cause tan spot of winter wheat. Cereal Research Communications. doi: 10.1007/s42976-021-00175-9
Kamel S, Cherif M, Hafez M, et al. (2019) Pyrenophora tritici–repentis in Tunisia: Race Structure and Effector Genes. Front. Plant Sci. 10: 1562. doi: 10.3389/fpls.2019.01562
Kaneps J, Bankina B, Moročko-Bičevska I (2021) Virulence of Pyrenophora tritici-repentis: a minireview. Agricultural Sciences. doi: 10.22616/rrd.27.2021.003
Kariyawasam GK, Carter AH, Rasmussen JB, et al. (2016) Genetic relationships between race-nonspecific and race-specific interactions in the wheat–Pyrenophora tritici–repentis pathosystem. Theor Appl Genet 129: 897–908. doi: 10.1186/s12864-018-4680-3
Kariyawasam GK, Wyatt N, Shi G, et al. (2021) A genome-wide genetic linkage map and reference quality genome sequence for a new race in the wheat pathogen Pyrenophora tritici-repentis. Fungal Genetics and Biology 152: 103571. doi: 10.1016/j.fgb.2021.103571
Karmacharya A, Li D, Leng Y, et al. (2023) Targeting Disease Susceptibility Genes in Wheat Through wide Hybridization with Maize Expressing Cas9 and Guide RNA. Mol Plant Microbe Interact. 36(9): 554-557. doi: 10.1094/MPMI-01-23-0004-SC
Kastelein P, Köhl J, Gerlagh M, Goossen-van de Geijn HM (2002) Inoculum sources of the tan spot fungus Pyrenophora tritici-repentis in The Netherlands. Meded Rijksuniv Gent Fak Landbouwkd Toegep Biol Wet. 67(2): 257-67.
Kim YM, Strelkov SE (2007) Heterologous expression and activity of Ptr ToxB from virulent and avirulent isolates of Pyrenophora tritici-repentis. Canadian Journal of Plant Pathology 29(3): 232-242. doi: 10.1080/07060660709507465
Kim YM, Bouras N, Kav NN, Strelkov SE (2010) Inhibition of photosynthesis and modification of the wheat leaf proteome by Ptr ToxB: a host‐specific toxin from the fungal pathogen Pyrenophora tritici‐repentis. Proteomics 10(16): 2911-2926. doi: 10.1002/pmic.200900670
Kokhmetova A, Kremneva O, Volkova G, et al. (2017) Evaluation of wheat cultivars growing in Kazakhstan and Russia for resistance to tan spot. J. Plant Pathol. 99: 161-167. doi: 10.4454/jpp.v99i1.3812
Kokhmetova AM, Kovalenko NM, Kumarbaeva MT (2020) Pyrenophora tritici-repentis population structure in the Republic of Kazakhstan and identification of wheat germplasm resistant to tan spot. Vavilovskii Zhurnal Genet Selektsii. 24(7): 722-729. doi: 10.18699/VJ20.666
Kokhmetova A, Sehgal D, Ali S, et al. (2021) Genome-Wide Association Study of Tan Spot Resistance in a Hexaploid Wheat Collection From Kazakhstan. Front. Genet. 11:581214. doi: 10.3389/fgene.2020.581214
Kremneva OY, Volkova GV, Kovalenko NM (2019) The dynamics of the race structure of Pyrenophora tritici-repentis in the North Caucasus Region. Mikologiya I Fitopatologiya 53: 246-253. doi: 10.1134/S0026364819040056
Kremneva OY, Mironenko NV, Volkova GV, et al. (2021) Resistance of winter wheat varieties to tan spot in the North Caucasus region of Russia. Saudi Journal of Biological Sciences 28: 1787-1794. doi: 10.1016/j.sjbs.2020.12.021
Kumarbayeva MK, Kovalenko NM, Kremneva OYu, et al. (2022) Identification of Wheat Samples for Resistance to Toxins of Pyrenophora tritici-repentis (Ptr). International Journal of Biology and Chemistry 15(1): 64-72. doi: 10.26577/ijbch.2022.v15.i1.07
Krupinsky JM (1992) Collection of conidia and ascospores of Pyrenophora tritici-repentis from wheat straw. Pages 91-95 in: Advances in Tan Spot Research. Proc. Int. Tan Spot Workshop, 2nd. L. J. Francl, J. M. Krupinsky, and M. P. McMuller, eds. N.D. Agric. Exp. Stn., Fargo.
Lamari L, Bernier CC (1994) Temperature-induced resistance to tan spot [Pyrenophora tritici-repentis] of wheat. Canadian Journal of Plant Pathology 16: 279-286. doi: 10.1080/07060669409500732
Lamari L, Gilbert J, Tekauz A (1998) Race differentiation in Pyrenophora tritici-repentis and survey of physiological variation in western Canada. Can. J. Plant Pathol. 20, 396–400. doi: 10.1080/07060669809500410
Lamari L, Strelkov SE, Yahyaoui A, et al. (2003) The identification of two new races of Pyrenophora tritici-repentis from the host center of diversity confirms a one-to-one relationship in tan spot of wheat. Phytopathology 93(4): 391-396. doi: 10.1094/PHYTO.2003.93.4.391
Lamari L, Strelkov SE (2010) The wheat/Pyrenophora tritici-repentis interaction: progress towards an understanding of tan spot disease. Canadian Journal of Plant Pathology 32(1): 4-10. doi: 10.1080/07060661003594117
Laribi M, Akhavan A, M’Barek S, et al. (2021) Characterization of Pyrenophora tritici-repentis in Tunisia and Comparison with a Global Pathogen Population. Plant Disease. doi: 10.1094/PDIS-04-21-0763-RE
Laribi M, Ben M’Barek S, Fakhfakh M, et al. (2021) Durum Wheat Mediterranean Landraces: A Valuable Source for Resistance to Tan Spot Disease. Agriculture 11(11): 1148. doi: 10.3390/agriculture11111148
Laribi M, Yahyaoui AH, Abdedayem W, et al. (2022) Characterization of Mediterranean Durum Wheat for Resistance to Pyrenophora tritici-repentis. Genes 13(2): 336. doi: 10.3390/genes13020336
Laribi M, Akhavan A, Ben M’Barek S, et al. (2022) Characterization of Pyrenophora tritici-repentis in Tunisia and Comparison with a Global Pathogen Population. Plant Dis. 106(2): 464-474. doi: 10.1094/PDIS-04-21-0763-RE
Larran S, Simón MR, Moreno MV, et al. (2016) Endophytes from wheat as biocontrol agents against tan spot disease. Biological Control 92: 17-23. doi: 10.1016/j.biocontrol.2015.09.002
Lepoint P, Renard ME, Legrève A, et al. (2010) Genetic diversity of the mating type and toxin production genes in Pyrenophora tritici-repentis. Phytopathology 100(5): 474-83. doi: 10.1094/PHYTO-100-5-0474
Liu Z, Zurn JD, Kariyawasam G, et al. (2017) Inverse gene-for-gene interactions contribute additively to tan spot susceptibility in wheat. Theor. Appl. Genet. 130: 1267-1276. doi: 10.1007/s00122-017-2886-4
Liu Y, Zhang Q, Salsman E, et al. (2020) QTL mapping of resistance to tan spot induced by race 2 of Pyrenophora tritici-repentis in tetraploid wheat. Theoretical and Applied Genetics 133(2): 433‐442. doi: 10.1007/s00122-019-03474-2
Manning VA, Chu AL, Steeves JE, et al. (2009) A host-selective toxin of Pyrenophora tritici-repentis, Ptr ToxA, induces photosystem changes and reactive oxygen species accumulation in sensitive wheat. Mol Plant Microbe Interact. 22(6): 665-676. doi: 10.1094/MPMI-22-6-0665
Manning VA, Pandelova I, Dhillon B, et al. (2013) Comparative genomics of a plant-pathogenic fungus, Pyrenophora tritici-repentis, reveals transduplication and the impact of repeat elements on pathogenicity and population divergence. G3 (Bethesda) 3: 41-63. doi: 10.1534/g3.112.004044
Manning VA, Ciuffetti LM (2015) Necrotrophic Effector Epistasis in the Pyrenophora tritici-repentis-Wheat Interaction. PLoS ONE 10(4): e0123548. doi: 10.1371/journal.pone.0123548
Martinez JP, Oesch NW, Ciuffetti LM (2004) Characterization of the multiple-copy host-selective toxin gene, ToxB, in pathogenic and nonpathogenic isolates of Pyrenophora tritici-repentis. Molecular Plant-Microbe Interactions 17(5): 467-474. doi: 10.1094/MPMI.2004.17.5.467
Martinez JP, Ottum SA, Ali S, et al. (2001) Characterization of the ToxB gene from Pyrenophora tritici-repentis. Molecular plant-microbe interactions 14(5): 675-677. doi: 10.1094/MPMI.2001.14.5.675
Masi M, Zorrilla JG, Meyer S (2022) Bioactive Metabolite Production in the Genus Pyrenophora (Pleosporaceae, Pleosporales). Toxins (Basel) 14(9): 588. doi: 10.3390/toxins14090588
Matsuzaki Y, Uda Y, Harada T, et al. (2022) Metyltetraprole activity against plant pathogens with relatively rare cytochrome b haplotypes for azoxystrobin resistance. J Gen Plant Pathol 88: 318–324. doi: 10.1007/s10327-022-01081-6
Maulenbay A, Zakarya K, Moldazhanova R, Rsaliyev A (2022) Characterization of Tan Spot Races in Kazakhstan. Agriculture 12(10): 1564. doi: 10.3390/agriculture12101564
McDonald MC, Oliver RP, Friesen TL, et al. (2013) Global diversity and distribution of three necrotrophic effectors in Phaeosphaeria nodorum and related species. New Phytol, 199: 241-251. doi: 10.1111/nph.12257
McDonald MC, Taranto AP, Hill E, et al. (2019) Transposon-Mediated Horizontal Transfer of the Host-Specific Virulence Protein ToxA between Three Fungal Wheat Pathogens. mBio. 10(5): e01515-19. doi: 10.1128/mBio.01515-19
MacLean DE, Aboukhaddour R, Tran VA, et al. (2017) Race characterization of Pyrenophora tritici-repentis and sensitivity to propiconazole and pyraclostrobin fungicides. Canadian Journal of Plant Pathology 39(4). doi: 10.1080/07060661.2017.1387178
Manguel DFL (2022) Characterization of the necrotrophic effector ToxA in Kansas fungal leaf spot pathogen populations. PhD thesis, Kansas State University. Link
Meinhardt SW, Cheng W, Kwon CY, et al. (2002) Role of the arginyl-glycyl-aspartic motif in the action of Ptr ToxA produced by Pyrenophora tritici-repentis. Plant Physiol. 130(3): 1545-51. doi: 10.1104/pp.006684
Mironenko NV, Orina AS, Kovalenko NM (2020) Differences between Pyrenophora tritici-repentis Isolates in Expression of ToxA and PtrPf2 Genes in Culture (in vitro). Russ J Genet 56: 509–512. doi: 10.1134/S1022795420040080
Mironenko NV, Orina AS, Kovalenko NM (2025) First Detection of the toxb2 Gene in Pyrenophora tritici-repentis Strains from Russia and Kazakhstan. Russ J Genet 61: 244–247. doi: 10.1134/S1022795424701606
Moffat CS, Stoll T, Moolhuijzen P (2018) Proteomics of the wheat tan spot pathogen Pyrenophora tritici-repentis. BMC Res Notes 11, 846. doi: 10.1186/s13104-018-3936-y
Momeni H, Aboukhaddour R, Javan-Nikkhah M (2014) Race identification of Pyrenophora tritici-repentis in Iran. Journal of Plant Pathology 96. doi: 10.4454/JPP.V96I2.036
Moolhuijzen P, See PT, Hane JK, et al. (2018) Comparative genomics of the wheat fungal pathogen Pyrenophora tritici-repentis reveals chromosomal variations and genome plasticity. BMC Genomics 19: 279. doi: 10.1186/s12864-018-4680-3
Moolhuijzen PM, See PT, Oliver RP, Moffat CS (2018) Genomic distribution of a novel Pyrenophora tritici-repentis ToxA insertion element. PLoS ONE 13(10): e0206586. doi: 10.1371/journal.pone.0206586
Moolhuijzen P, See PT, Moffat CS (2019) A new PacBio genome sequence of an Australian Pyrenophora tritici-repentis race 1 isolate. BMC Res. Notes 12: 642. doi: 10.1186/s13104-019-4681-6
Moolhuijzen P, See PT, Moffat CS (2021) The first genome assembly of fungal pathogen Pyrenophora tritici-repentis race 1 isolate using Oxford Nanopore MinION sequencing. BMC Res Notes 14: 334. doi: 10.1186/s13104-021-05751-0
Moolhuijzen PM, See PT, Shi G, et al. (2022) A global pangenome for the wheat fungal pathogen Pyrenophora tritici-repentis and prediction of effector protein structural homology. Microb Genom. 8(10): mgen000872. doi:
Moreno MV, Stenglein SA, Balatti PA, Perelló AE (2008) Pathogenic and molecular variability among isolates of Pyrenophora tritici-repentis, causal agent of tan spot of wheat in Argentina. European Journal of Plant Pathology 122(2): 239–252. doi: 10.1007/s10658-008-9277-2
Moreno MV, Stenglein S, Perelló A (2012) Pyrenophora tritici-repentis, Causal Agent of Tan Spot: A Review of Intraspecific Genetic Diversity. In book: The Molecular Basis of Plant Genetic Diversity. doi: 10.5772/33516
Moreno MV, Stenglein S, Perelló AE (2015) Distribution of races and Tox genes in Pyrenophora tritici-repentis isolates from wheat in Argentina. Tropical Plant Pathology 40(2): 141-146. doi: 10.1007/s40858-015-0011-2
Muqaddasi QH, Kamal R, Mirdita V, et al. (2021) Genome-Wide Association Studies and Prediction of Tan Spot (Pyrenophoratritici-repentis) Infection in European Winter Wheat via Different Marker Platforms. Genes (Basel) 12(4): 490. doi: 10.3390/genes12040490
Nisikado Y (1928) Preliminary Notes on Yellow Spot Disease of Wheat Caused by Helminthosporium Tritici-vulgaris Nisikado. Berichte des Ohara Instituts für landwirtschaftliche Forschungen 4(1): 103-109.
Nisikado Y (1928) Preliminary notes on a new helminthosporiose of wheat (Triticum vulgare Vill.). Japanese Journal of Phytopathology 2(2): 89-98_2.
Nyarko A, Singarapu KK, Figueroa M, et al. (2014) Solution NMR structures of Pyrenophora tritici-repentis ToxB and its inactive homolog reveal potential determinants of toxin activity. J Biol Chem. 289(37): 25946-56. doi: 10.1074/jbc.M114.569103
Oliver RP, Solomon PS (2008) Recent fungal diseases of crop plants: is lateral gene transfer a common theme? Mol Plant Microbe Interact. 21(3): 287-93. doi: 10.1094/MPMI-21-3-0287
Oliver RP, Lord M, Rybak K, et al. (2008) Emergence of tan spot disease caused by toxigenic Pyrenophora tritici-repentis in Australia is not associated with increased deployment of toxin-sensitive cultivars. Phytopathology 98(5): 488-91. doi: 10.1094/PHYTO-98-5-0488
Pandelova I, Figueroa M, Wilhelm LJ, et al. (2012) Host-Selective Toxins of Pyrenophora tritici-repentis Induce Common Responses Associated with Host Susceptibility. PLoS ONE 7(7): e40240. doi: 10.1371/journal.pone.0040240
Patel JS, Gudmestad NC, Meinhardt S, et al. (2012) Pyraclostrobin sensitivity of baseline and fungicide exposed isolates of Pyrenophora tritici-repentis. Crop Protection 34: 37-41. doi: 10.1016/j.cropro.2011.10.015
Patel JS, Mamidi S, Bonman JM, Adhikari TB (2013) Identification of QTL in Spring Wheat Associated with Resistance to a Novel Isolate of Pyrenophora tritici-repentis. Crop Science 53: 842-852. doi: 10.2135/cropsci2012.01.0036
Pazdiora PC, da Rosa Dorneles K, Morello TN, et al. (2021) Silicon soil amendment as a complement to manage tan spot and fusarium head blight in wheat. Agronomy for Sustainable Development, 41, 21. doi: 10.1007/s13593-021-00677-0
Peters Haugrud AR, Shi G, Seneviratne S, et al. (2023) Genome-wide association mapping of resistance to the foliar diseases septoria nodorum blotch and tan spot in a global winter wheat collection. PREPRINT (Version 1) available at Research Square doi: 10.21203/rs.3.rs-2557769/v1
Pfender WF, Wootke SL (198). Production of pseudothecia and ascospores by Pyrenophora tritici-repentis in response to macronutrient concentrations. Phytopathology 77: 1213-1216. doi: 10.1094/Phyto-77-1213
Pfender F, Zhang W, Nus A (1993) Biological Control to Reduce Inoculum of the Tan Spot Pathogen Pyrenophora tritici-repentis in Surface-borne Residues of Wheat Fields. Phytopathology 83: 371-375. doi: 10.1094/Phyto-83-371
Prahl KC, Klink H, Hasler M, et al. (2022) Can Decision Support Systems Help Improve the Sustainable Use of Fungicides in Wheat? Sustainability 14(23): 15599. doi: 10.3390/su142315599
Rees RG, Platz GJ (1990) Sources of resistance to Pyrenophora tritici-repentis in bread wheats. Euphytica 45: 59–69. doi: 10.1007/BF00032151
Reis EM, Zanatta M, Forcelini CA (2019) Addition of mancozeb to the fungicide mixtures DMI + QoI and SDHI + QoI on the control of wheat leaf blights. Summa Phytopathologica 45: 23-27. doi: 10.1590/0100-5405/179192
Reynoso A, Sautua F, Carmona M, et al. (2023) Tan spot of wheat: can biological control interact with actual management practices to counteract this global disease?. Eur J Plant Pathol. doi: 10.1007/s10658-023-02647-6
Román Ramos AE, Kutcher HR, Dallagnol LJ (2023) Pyrenophora tritici-repentis: A Worldwide Threat to Wheat. In: Otinga Wanyera R, Wamalwa M (eds), Wheat. IntechOpen. doi: 10.5772/intechopen.110306
Różewicz M, Wyzińska M, Grabiński J (2021) The Most Important Fungal Diseases of Cereals—Problems and Possible Solutions. Agronomy 11(4): 714. doi: 10.3390/agronomy11040714
Sah DN (1994) Effects of Leaf Wetness Duration and Inoculum Level on Resistance of Wheat Genotypes to Pyrenophora tritici-repentis. Journal of Phytopathology 142: 324-330. doi: 10.1111/j.1439-0434.1994.tb04546.x
Sarma GN, Manning VA, Ciuffetti LM, Karplus PA (2005) Structure of Ptr ToxA: an RGD-containing host-selective toxin from Pyrenophora tritici-repentis. Plant Cell. 17(11): 3190-202. doi: 10.1105/tpc.105.034918
Sautua FJ, Carmona MA (2021) Detection and characterization of QoI resistance in Pyrenophora tritici-repentis populations causing tan spot of wheat in Argentina. Plant Pathology 70: 2125–2136. doi: 10.1111/ppa.13436
Sautua FJ, Carmona MA (2022) Baseline sensitivity of QoI-resistant isolates of Pyrenophora tritici-repentis from Argentina to fenpicoxamid. Eur J Plant Pathol . doi: 10.1007/s10658-022-02582-y
Schierenbeck M, Fleitas MC, Gerard GS, et al. (2019) Combinations of fungicide molecules and nitrogen fertilization revert nitrogen yield reductions generated by Pyrenophora tritici-repentis infections in bread wheat. Crop Protection 121: 173-181. doi: 10.1016/j.cropro.2019.04.004
Schierenbeck M, Fleitas MC, Simón MR (2022) The Interaction of Fungicide and Nitrogen for Aboveground Biomass from Flag Leaf Emergence and Grain Yield Generation under Tan Spot Infection in Wheat. Plants 12(1): 212. doi: 10.3390/plants12010212
Schilder AM, Bergstrom GC. In: Francl LJ, Krupinsky JM, McMullen MP (1992) The dispersal of conidia and ascospores of Pyrenophora tritici-repentis; Proceedings of 2nd Tan Spot Workshop; Fargo. pp. 96–99.
Schilder AMC, Bergstrom GC (1995) Seed transmission of Pyrenophora tritici-repentis, causal fungus of tan spot of wheat. European Journal of Plant Pathology 101: 81–91. doi: 10.1007/BF01876096
Schuh W (1990) The influence of tillage systems on incidence and spatial pattern of tan spot of wheat. Phytopathol 80: 804–807.
Schutt de Varini LS, Formento AN, Velázquez PD, Velázquez JC (2013) Comportamiento de cultivares de trigo a mancha amarilla (Drechslera tritici-repentis) en lotes con diferentes antecesores. Revista Agromercado. Cuadernillo Clásico de Trigo Nº 174. pp. 21-24. ISSN 1515-223X
See PT, Moffat CS, Morina J, Oliver RP (2016) Evaluation of a multi-locus indel DNA region for the detection of the wheat tan spot pathogen Pyrenophora tritici-repentis. Plant Disease 100(11): 2215-2225. doi: 10.1094/PDIS-03-16-0262-RE
See PT, Marathamuthu KA, Iagallo EM, et al. (2018) Evaluating the importance of the tan spot ToxA–Tsn1 interaction in Australian wheat varieties. Plant Pathology (accepted). doi: 10.1111/ppa.12835
See PT, Schultz N, Moffat CS (2020) Evaluation of Pyrenophora tritici-repentis Infection of Wheat Heads. Agriculture 10(9):417. doi: 10.3390/agriculture10090417
, , , et al. (2021) Virulence assessment of Australian Pyrenophora tritici-repentis isolates. Plant Pathology 00: 1– 10. doi: 10.1111/ppa.13484
See PT, Moffat CS (2021) Evaluation of a Novel Molecular Marker Associated with the Tan Spot Disease Response in Wheat. Agriculture 11(6): 513. doi: 10.3390/agriculture11060513
Semagn K, Iqbal M, Jarquin D, et al. (2022) Genomic Predictions for Common Bunt, FHB, Stripe Rust, Leaf Rust, and Leaf Spotting Resistance in Spring Wheat. Genes 13(4): 565. doi: 10.3390/genes13040565
Shaukat S, Francl LJ (2002) Race structure of Pyrenophora tritici-repentis isolates obtained from wheat in South America. Plant Prot. Sci. 38: 302–304. Link
Shankar M, Jorgensen D, Taylor J, et al. (2017) Loci on chromosomes 1A and 2A afect resistance to tan (yellow) spot in wheat populations not segregating for tsn1. Theoretical and Applied Genetics 130(12): 2637–2654. doi: 10.1007/s00122-017-2981-6
Shi GS, Kariyawasam G, Liu S, et al. (2022) A conserved hypothetical gene is required but not sufficient for Ptr ToxC production in Pyrenophora tritici-repentis. Mol Plant Microbe Interact. doi: 10.1094/MPMI-12-21-0299-R
Sierotzki H, Frey R, Wullschleger J, et al. (2007) Cytochrome b gene sequence and structure of Pyrenophora teres and P. tritici-repentis and implications for QoI resistance. Pest Management Science 63: 225–233. doi: 10.1002/ps.1330
Singh S, Bockus WW, Sharma I, Bowden RL (2008) A Novel Source of Resistance in Wheat to Pyrenophora tritici-repentis Race 1. Plant Disease 92(1): 91‐95. doi: 10.1094/PDIS-92-1-0091
Singh PK, Mergoum M, Ali S, et al. (2008) Genetic analysis of resistance to Pyrenophora tritici-repentis races 1 and 5 in tetraploid and hexaploid wheat. Phytopathology. 98(6): 702‐708. doi: 10.1094/PHYTO-98-6-0702
Singh PK, Singh RP, Duveiller E, et al. (2010) Genetics of wheat–Pyrenophora tritici-repentis interactions. Euphytica 171: 1-13. doi: 10.1007/s10681-009-0074-6
Sisson AJ, Allen T, Andersen K, et al. (2025) Estimated yield reductions and economic losses on wheat caused by disease from 2018 through 2021. Plant Health Progress. doi: 10.1094/PHP-09-24-0087-RS
Strelkov SE (1997) Purification and mode of action of PTR, Pyrenophora tritici-repentis, chlorosis toxin. Master’s thesis, University of Manitoba. Link
Strelkov SE, Lamari L, Ballance GM (1998) Induced chlorophyll degradation by a chlorosis toxin from Pyrenophora tritici-repentis. Canadian journal of plant pathology 20(4): 428-435. doi: 10.1080/07060669809500417
Strelkov SE, Lamari L, Ballance GM (1999) Characterization of a host-specific protein toxin (Ptr ToxB) from Pyrenophora tritici-repentis. Molecular Plant-Microbe Interactions 12(8): 728-732. doi: 10.1094/MPMI.1999.12.8.728
Strelkov SE, Lamari L (2003) Host–parasite interactions in tan spot [Pyrenophora tritici-repentis] of wheat. Canadian Journal of Plant Pathology 25(4): 339-349. doi: 10.1080/07060660309507089
Strelkov SE, Kowatsch RF, Ballance GM, Lamari L (2005) Characterization of the ToxB gene from North African and Canadian isolates of Pyrenophora tritici-repentis. Physiological and Molecular Plant Pathology 67(3-5): 164-170. doi: 10.1016/j.pmpp.2005.12.004
Stukenbrock EH, McDonald BA (2007) Geographical variation and positive diversifying selection in the host‐specific toxin SnToxA. Molecular Plant Pathology 8(3): pp.321-332. doi: 10.1111/j.1364-3703.2007.00396.x
Summerell BA, Burgess LW (1988) Saprophytic colonization of wheat and barley by Pyrenophora tritici-repentis in the field. Transactions of the British Mycological Society 90(4): 551-556. doi: 10.1016/S0007-1536(88)80058-5
Summerell BA, Burgess LW (1988) Factors influencing production of pseudothecia by Pyrenophora tritici-repentis. Transactions of the British Mycological Society 90: 557-562. doi: 10.1016/S0007-1536(88)80059-7
Sun XC, Bockus W, Bai G (2010) Quantitative trait loci for resistance to Pyrenophora tritici-repentis race 1 in a Chinese wheat. Phytopathology 100: 468-473. doi: 10.1094/PHYTO-100-5-0468
Sun B-F, Xiao J-H, He S, et al. (2013) Multiple Interkingdom Horizontal Gene Transfers in Pyrenophora and Closely Related Species and Their Contributions to Phytopathogenic Lifestyles. PLoS ONE 8(3): e60029. doi: 10.1371/journal.pone.0060029
Tadesse W, Hsam SLK, Zeller FJ (2006) Evaluation of common wheat cultivars for tan spot resistance and chromosomal location of a resistance gene in the cultivar ‘Salamouni’. Plant Breeding 125: 318-322. doi: 10.1111/j.1439-0523.2006.01243.x
Tissaoui S, Hassine M, Mougou-Hamdane A, et al. (2022) Varietal Screening of Durum Wheat Varieties for Resistance to Pyrenophora tritici-repentis (Tan Spot) under Field Conditions. BioMed Research International, vol. 2022, Article ID 6433577. doi: 10.1155/2022/6433577
Tonin RB, Ranzi C, Camera JN, et al. (2014) Amplitude térmica para germinação de conídios de Drechslera tritici-repentis. Summa Phytopathologica 40(2): 174-177. doi: 10.1590/0100-5405/1881
Tonin RB, Reis EM, Avozani A (2017) Reduction in the in vitro sensitivity of Drechslera tritici-repentis, isolated from wheat, to strobilurin and triazole fungicides. Summa Phytopathologica 43(1): 20-25. doi: 10.1590/0100-5405/2160
Tran VA, Aboukhaddour R, Strelkov IS, et al. (2017) The sensitivity of Canadian wheat genotypes to the necrotrophic effectors produced by Pyrenophora tritici-repentis. Canadian Journal of Plant Pathology 39: 149-162. doi: 10.1080/07060661.2017.1339125
Valder PG, Shaw DE (1953) Yellow spot disease of wheat in Australia. Proc. Linn. Soc. N. S. W. 77, 323–330.
Vidal T, Lusley P, Leconte M, et al. (2017) Cultivar architecture modulates spore dispersal by rain splash: A new perspective to reduce disease progression in cultivar mixtures. PLoS ONE 12(11): e0187788. doi: 10.1371/journal.pone.0187788
Virdi SK, Liu Z, Overlander ME, et al. (2016) New insights into the roles of host gene-necrotrophic effector interactions in governing susceptibility of durum wheat to tan spot and Septoria nodorum blotch. G3: GENES, GENOMES, GENETICS 6(12): 4139-4150. doi: 10.1534/g3.116.036525
Wegulo SN (2011) Tan spot of cereals. The Plant Health Instructor. doi: 10.1094/PHI-I-2011-0426-01
Wegulo SN, Klein RN, Harveson RM (2012) Tan Spot of Wheat. University of Nebraska. Link
Wei B, Moscou MJ, Sato K, et al. (2020) Identification of a Locus Conferring Dominant Susceptibility to Pyrenophora tritici-repentis in Barley. Front. Plant Sci. 11:158. doi: 10.3389/fpls.2020.00158
Wei B, Despins T, Fernandez MR, et al. (2021) Race Distribution of Pyrenophora tritici-repentis in Relation to Ploidy Level and Susceptibility of Durum and Winter Bread Wheat, Canadian Journal of Plant Pathology, doi: 10.1080/07060661.2020.1870002
Weith SK (2015) Pyrenophora tritici-repentis the causal agent of tan spot: characterisation of New Zealand populations. Master thesis, Lincoln University. Link
Wright KH, Sutton JC (1990) Inoculum of Pyrenophora tritici-repentis in relation to epidemics of tan spot of winter wheat in Ontario. Canadian Journal of Plant Pathology 12(2): 149-157. doi: 10.1080/07060669009501018