.
Condición fitosanitaria: Presente
Grupo de cultivos: Cereales
Especie hospedante: Arroz (Oryza sativa)
Rango de hospedantes: M. oryzae tiene un rango amplio de hospedantes, pero los aislados individuales tienen rangos de hospedantes limitados y se dividen en diferentes patotipos según su compatibilidad (Chung et al., 2020).
Epidemiología: policíclica, subaguda.
Etiología: Hongo. Hemibiotrófico
Agente causal: Magnaporthe oryzae (anamorph: Pyricularia oryzae Cavara, 1892)
Taxonomía: Eukaryota > Fungi > Dikarya > Ascomycota > Pezizomycotina > Sordariomycetes > Magnaporthales > Magnaporthe
.
.
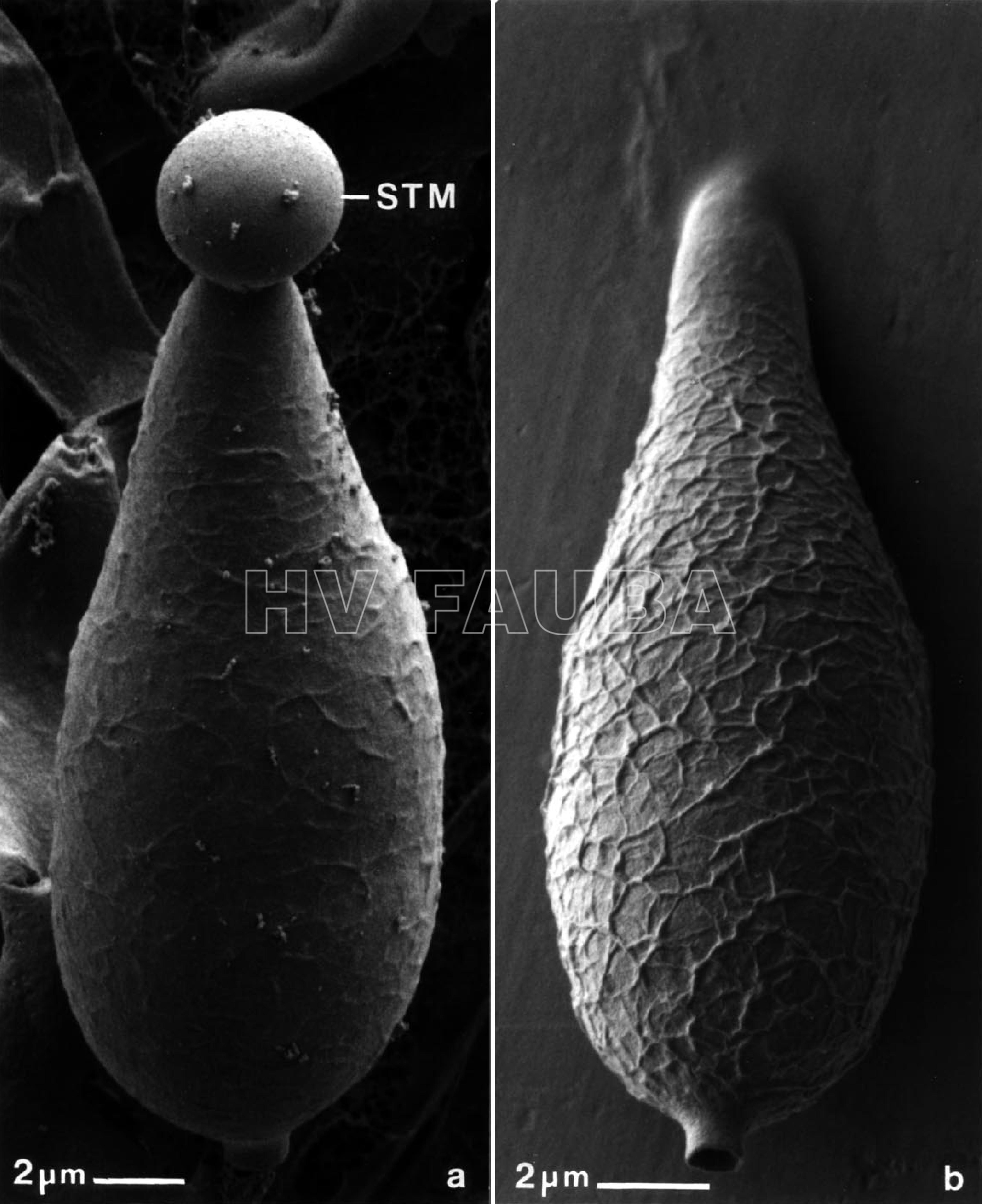
- Micrografías electrónicas de barrido criogénico de conidios de M. grisea. (a) Conidio soportado en el conidióforo, que exhibe una gota apical de mucílago adhesivo en la punta de la espora (STM). (b) La fijación de conidio por medio de STM resultó de tocar la superficie del cubreobjetos de plástico a la colonia esporulante. Nótese la ornamentación reticulada única en la superficie de los conidios. Autor: Howard y Valent, 1996.
.
.
Sintomatología
Pyricularia oryzae ataca hojas, tallos, inflorescencias y ocasionalmente al grano. Los momentos, en que la planta de arroz es más susceptible, son el estado de plántula y durante la floración, incluso las raíces pueden infectarse. Sin embargo, el síntoma más común y que sirve como diagnóstico, son lesiones en forma de diamante que ocurre en las hojas, mientras que las lesiones en las vainas son relativamente raras.
Hojas: los síntomas en las hojas pueden variar de acuerdo con las condiciones ambientales, la edad de la planta y los niveles de resistencia de los cultivares hospedantes. En cultivares susceptibles, las lesiones pueden aparecer inicialmente con un color verde grisáceo, con un borde verde más oscuro y se expanden rápidamente a varios centímetros de longitud. En cultivares susceptibles, las lesiones más viejas a menudo adquieren un color tostado claro con bordes necróticos. En cultivares resistentes, las lesiones suelen ser pequeñas en tamaño (1-2 mm) y de color café a marrón oscuro.
Unión de la hoja y la vaina del tallo: los síntomas de infección consisten en un área general de necrosis en la unión de los dos tejidos. Este tipo de infecciones pueden matar toda la hoja y extenderse unos pocos milímetros dentro y alrededor de la cubierta. El hongo puede producir esporas en estas lesiones.
Cuellos y panículas: el cuello de la planta de arroz se refiere a la porción del tallo que se eleva sobre las hojas y sostiene la cabeza o la panícula de la semilla. Los cuellos a menudo se infectan en el nódulo y la infección conduce a una afección llamada cuello podrido o explosión del cuello. La infección de los cuellos puede ser muy destructiva, causando que las semillas no se llenen, o haciendo que toda la panícula se caiga como si estuviera podrida. El hongo también puede infectar las panículas a medida que se forman las semillas. Las lesiones se pueden encontrar en las ramas de la panícula, espinas y espiguillas. Las lesiones a menudo son decoloraciones de color marrón grisáceo de las ramas de la panícula y, con el tiempo, las ramas pueden romperse en la lesión.
Cuando la infección se inicia en un nudo, éste toma un color grisáceo y la parte superior de la planta se seca. Esta zona suele ser un punto de rotura de la caña.
Semillas: el hongo a menudo se ha aislado de los pedicelos de las semillas. Las semillas no se producen cuando los pedicelos se infectan, una condición llamada cegamiento. Los síntomas de la explosión del arroz en las semillas mismas consisten en manchas marrones, y ocasionalmente, la lesión clásica en forma de diamante de las hojas. El proceso y el tiempo durante el cual ocurre la infección de las semillas por las esporas del patógeno no se ha descrito completamente, pero la información reciente muestra que el hongo puede infectar las semillas infectando las flores mientras maduran en semillas, y se cree que este es la principal manera en que se desarrolla la infección de semilla.
.
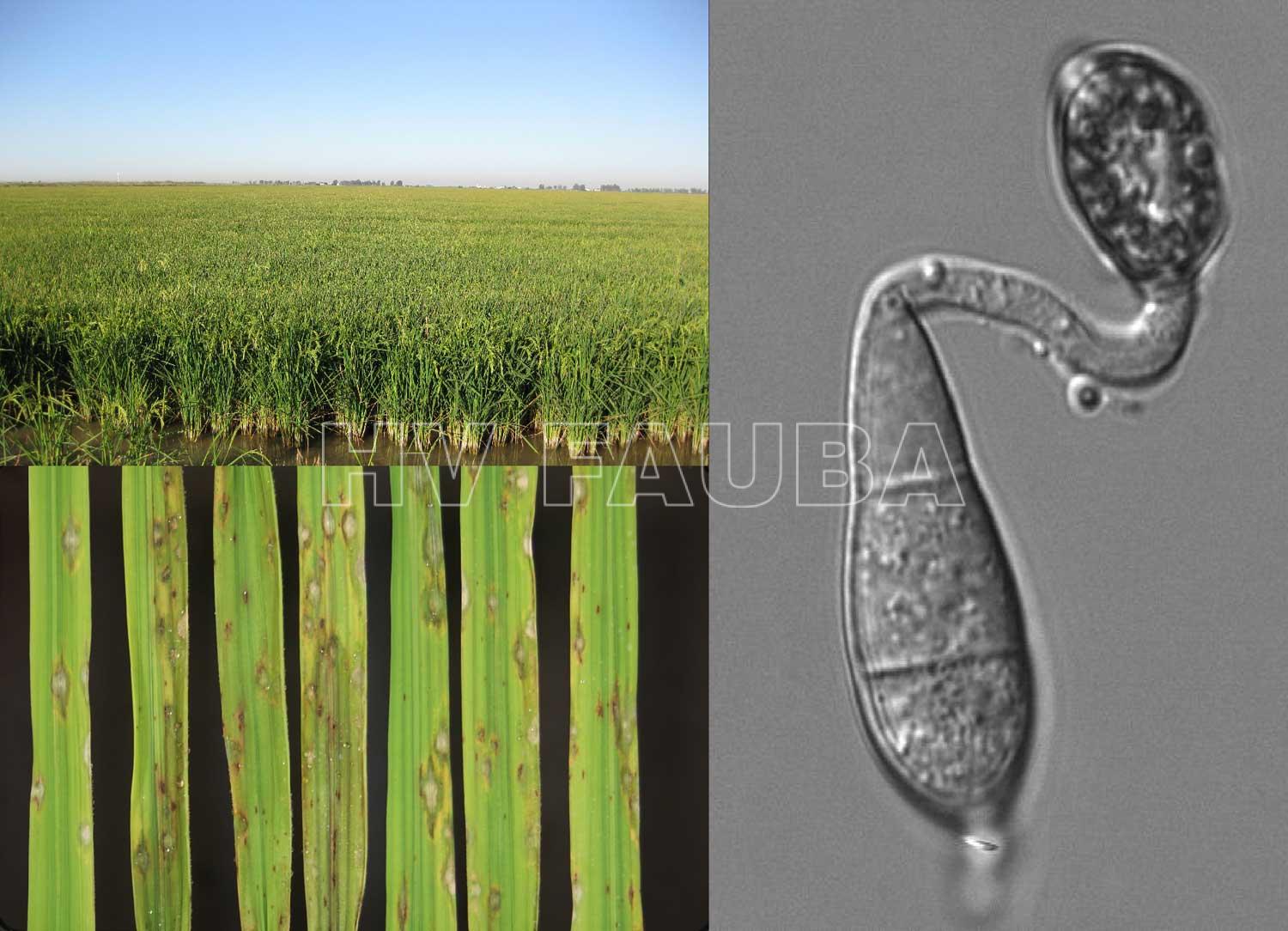
- Campo de arroz infectado con Magnaporthe oryzae. Conidio germinado con formación de apresorio. Autor: Julio L. Rodríguez Romero
.
Hospedantes
Afecta un amplio espectro de especies de las Gramíneas, pero el principal daño económico lo provoca en arroz.
.
Condiciones predisponentes
Alta humedad relativa (90 – 92 %), gran intensidad lumínica, periodos largos de fotoperiodismo, tiempo ventoso, temperaturas nocturnas rondando los 20 ºC alternando con temperaturas diurnas entre 30 – 35 ºC.
.
- Síntomas del tizón del arroz. Autor: Database for Rice Seed-borne Fungi
- (A) Esporulación de Pyricularia oryzae en lesión foliar. (B) Esporulación en WA. (C) Conidios. (D – F) Conidióforos y conidios (barras = 5 μm). Autor: Database for Rice Seed-borne Fungi
.
.
Ciclo de la enfermedad y epidemiología
M. oryzae produce esporas sexuales (ascosporas) en estructuras llamadas asci (ascos), y se clasifica en la familia Magnaporthaceae recientemente erigida. Los asci se encuentran dentro de estructuras especializadas llamadas peritecios. El micelio de M. oryzae está septado y los núcleos dentro del micelio y las esporas de este hongo son haploides.
Como ascomicete, produce ascosporas hialinas, de forma fusiforme (con forma de huso con extremos decrecientes) con tres tabiques. Los asci son unitunicados. Se considera que este hongo es heterotálico con un sistema de acoplamiento bipolar (apareamiento controlado por dos alelos diferentes en un único locus) con genes adicionales que controlan el ciclo sexual. Con base en datos filogenéticos, moleculares y morfológicos recientes, aislamientos del hongo del arroz y aislamientos estrechamente relacionados de otros pastos como Eragrostris curvula, Eleusine coracana, Lolium perenne y Setaria spp. se describen taxonómicamente como Magnaporthe oryzae, mientras que los aislamientos de Digitaria sanguinalis son distintos y deben describirse como Magnaporthe grisea.
La etapa asexual de Magnaporthe oryzae se describe con el nombre de Pyricularia oryzae (antes llamada P. grisea) y es la forma de espora más común del hongo en Argentina. Estas esporas, llamadas conidios, se producen abundantemente en lesiones y en cultivo en tallos especializados, llamados conidióforos. Los conidios generalmente son tricelulares y se producen en el ápice de un conidióforo. Las colonias esporuladas en placas de agar pueden adquirir una apariencia grisácea y velluda.
En condiciones favorables, el hongo esporula en el centro de las lesiones en cultivares susceptibles. También puede esporular en lesiones de semillas. Raramente esporula en los cultivares más resistentes. Las esporas se producen en hojas, collar, panículas y semillas infectadas, en conidióforos que se extienden más allá de las superficies lesionadas; los conidióforos y las esporas en masa pueden dar a las lesiones un aspecto gris polvoriento. Los conidios se producen después de varias horas de alta humedad y se liberan más fácilmente cerca del mediodía, especialmente en condiciones de viento. El viento puede llevar los conidios a distancias considerables.
La infección del arroz ocurre cuando los conidios se depositan en los tejidos de arroz y germinan al producir un tubo germinativo y un apresorio. El apresorio es una estructura melanizada, y de ella se desarrolla una clavija de infección que penetra el tejido. El hongo penetra directamente por la cutícula. Después de la penetración, la hifa de infección primaria crece rápidamente y se ramifica dentro de los tejidos susceptibles. El crecimiento dentro de los tejidos de los cultivares resistentes a menudo se inhibe.
En general, el tizón del arroz se ve favorecido por temperaturas moderadas (24°C) y períodos de alta humedad que son de 12 horas o más, condiciones fácilmente alcanzables en campos de arroz inundados.
Las fuentes de esporas invernantes que comprenden el inóculo primario consisten en hierbas, plantas voluntarias, rastrojos infestados y semillas infestadas en la superficie del suelo después de la siembra mecánica. Las semillas infestadas que quedan en la superficie del suelo pueden producir fácilmente esporas de P. oryzae durante más de varias semanas después de la siembra, mucho después de que hayan surgido las plántulas. En invernadero, el hongo también esporula sobre los coleópticos muertos o moribundos de plantas que crecen a partir de semillas infectadas.
Las esporas producidas como inóculo primario en los tejidos que invernan, producen las infecciones iniciales en plántulas jóvenes, al depositarse en las hojas, germinando e invadiendo los tejidos de las hojas. La gravedad de la enfermedad a menudo se correlaciona con la cantidad de material infestado (densidad de inóculo). Las lesiones en las plántulas jóvenes aparecen unos días después de la infección. Estas lesiones secundarias producen más esporas y estas esporas se diseminan fácilmente por el viento a los tejidos de hojas sanos cercanos. La esporulación ocurre cuando la humedad relativa alcanza al 100 %.
Los ciclos secundarios pueden repetirse muchas veces durante la temporada de crecimiento, con el potencial de generar una gran cantidad de infecciones dentro de la estación de cultivo. El número de ciclos y el número de esporas que se producen en cada lesión individual pueden verse influenciados por muchos factores, que incluyen la temperatura, la lluvia, la profundidad del agua en el arrozal, la cantidad de nitrógeno utilizada para fertilizar el arroz y la nivel de resistencia genética en el cultivar que está infectado.
En general, la enfermedad en las hojas es más severa cuando las temperaturas diarias son moderadas, y cuando el arroz está sobre-fertilizado, o si se cultiva en aguas de inundación por debajo de las profundidades recomendadas. Bajo estas condiciones propicias para la enfermedad, se han registrado muy altas incidencias de la enfermedad en cultivares susceptibles, en comparación con cultivares genéticamente resistentes al hongo.
.
.
.
Manejo Integrado de la enfermedad
* Rotación de cultivos
* Usar semilla libre de enfermedad
* No excederse en la fertilización nitrogenada
* Uso de fungicidas: los momentos de tratamiento que se consideran más eficaces son formación de espiga e inicio floración.
* Control biológico: se han reportado casos satisfactorios de biocontrol del patógeno (Sha et al., 2020; Zhu et al., 2021)
.
.
- 01 Lesión en hoja del tizón del arroz, causado por Pyricularia oryzae. Infección natural de O. sativa (arroz) Variedad GURI INTA CL. Autor: Ing. Agr. Lisandro Bastida
- 02 Lesiones en hoja del tizón del arroz, causado por Pyricularia oryzae. Ensayos de patogenicidad directa. Inóculo y hospedantes O. sativa (Arroz) Variedad GURI INTA CL. Autor: Ing. Agr. Lisandro Bastida
- 03 Lesiones en hoja del tizón del arroz, causado por Pyricularia oryzae. Ensayos de patogenicidad directa. Inóculo y hospedantes O. sativa (Arroz) Variedad GURI INTA CL. Autor: Ing. Agr. Lisandro Bastida
- 04 Infección de Pyricularia oryzae en O. sativa (Arroz) Variedad GURI INTA CL. Autor: Ing. Agr. Lisandro Bastida
- 05 Conidios de Pyricularia oryzae de Avena sativa. 100X. Autor: Ing. Agr. Lisandro Bastida
- 06 Colonia de de P. oryzae con 7 días de crecimiento en APG obtenida de E. indica. Autor: Ing. Agr. Lisandro Bastida
- 07 Lesiones de P. oryzae en Luziola peruviana. Autor: Ing. Agr. Lisandro Bastida
- 08 Lesiones de P. grisea en Digitaria ciliaris. Autor: Ing. Agr. Lisandro Bastida
.
.
.
Videos
What can we do to actively manage wheat blast?
Septin-Dependent Assembly of the Exocyst Is Essential for Plant Infection by Magnaporthe oryzae
Mitosis in the appressorium and invasive hypha of the rice blast fungus (www.khanglab.org)
.
.
Bibliografía
Acebo Guerrero Y, Hernández-Rodríguez A, Rives Rodríguez N, et al. (2011) Perspectivas del uso de bacterias rizosféricas en el control de Pyricularia grisea (Cooke Sacc.) en el cultivo del arroz (Oryza sativa L.). Revista Colombiana de Biotecnología 13(1): 16-22. ISSN electrónico 1909-8758
Akator SK, Adjata DK, Hode GY, et al. (2014) Cultural and pathological studies of Pyricularia oryzae Isolates at Abomey Calavi in Benin. Plant Pathology Journal 13: 44-49. doi: 10.3923/ppj.2014.44.49
Alqahtani O, Mirajkar KK, Kumar K R A, et al. (2022) In Vitro Antibacterial Activity of Green Synthesized Silver Nanoparticles Using Azadirachta indica Aqueous Leaf Extract against MDR Pathogens. Molecules 27(21): 7244. doi: 10.3390/molecules27217244
Anjago WM, Biregeya J, Mingyue S, et al. (2022) The protein phosphatases MoPtc1 and MoPtc2 are induced during pathogen-host interactions and play synergistic roles in regulating MAPK pathways in Magnaporthe oryzae. bioRxiv 2022.09.09.507255; doi: doi: 10.1101/2022.09.09.507255
Ascari JP, Del Ponte EM (2021) Taxonomic and pathogenic diversity of the blast pathogen populations infecting wheat and grasses in Minas Gerais. doi: 10.31219/osf.io/ydqsz
Barragan CA, Latorre SM, Malmgren A, et al. (2024) Multiple horizontal mini-chromosome transfers drive genome evolution of clonal blast fungus lineages. bioRxiv 2024.02.13.580079; doi: 10.1101/2024.02.13.580079
Bastida LM, Gutiérrez S, Carmona M (2017) Relevamiento de hospedantes cultivados y espontáneos de Pyricularia spp. en Corrientes. XIII Encuentro Nacional de Monitoreo. Universidad Nacional de Córdoba, Facultad de Ciencias Agropecuarias.
Bastida LM, Gutiérrez S, Carmona M (2017) Relevamiento de hospedantes espontáneos de Pyricularia spp. en Corrientes. XXIII Comunicaciones Científicas y Tecnológicas. Universidad Nacional del Nordeste.
Bastida LM, Gutiérrez S, Cabrera G (2016) Hongos asociados a vegetación silvestre en Corrientes. XXV Reunión de comunicaciones científicas, técnicas y de extensión. Universidad Nacional del Nordeste, Facultad de Ciencias Agrarias.
, , , et al. (2024) Pyricularia oryzae: Lab star and field scourge. Molecular Plant Pathology 25: e13449. doi: 10.1111/mpp.13449
Bentham AR, Petit-Houdenot Y, Win J, et al. (2021) A single amino acid polymorphism in a conserved effector of the multihost blast fungus pathogen expands host-target binding spectrum. PLoS Pathog 17(11): e1009957. doi: 10.1371/journal.ppat.1009957
Biswas B, Thakur K, Pote TD, et al. (2023) Genetic and molecular analysis of leaf blast resistance in Tetep derived line RIL4 and its relationship to genes at Pita/Pita2 locus. Sci Rep. 13(1): 18683. doi: 10.1038/s41598-023-46070-7
Bonman JM (1992) Rice Blast. In: Compendium of Rice Diseases. Eds. R.K. Webster and P.S. Gunnel. American Phytopathological Society Press. St. Paul, Minnesota. USA. Pages 14-18.
, , , et al. (2023) Barley MLA3 recognizes the host-specificity effector Pwl2 from Magnaporthe oryzae. The Plant Cell: koad266. doi: 10.1093/plcell/koad266
Bühring S, Brunner A, Heeb K, et al. (2024) An array of signal-specific MoYpd1 isoforms determines full virulence in the pathogenic fungus Magnaporthe oryzae. Commun Biol. 7(1): 265. doi: 10.1038/s42003-024-05941-z
Bussaban B, Lumyong S, Lumyong P, et al. (2017) Molecular and morphological characterization of Pyricularia and allied genera. Mycologia 97(5): 1002-1011. doi: 10.1080/15572536.2006.11832750
Cao Y, Lu M, Chen J, et al. (2024) Identification of Ossnrk1a-1 Regulated Genes Associated with Rice Immunity and Seed Set. Plants (Basel). 13(5): 596. doi: 10.3390/plants13050596
Cesari S, Xi Y, Declerck N, et al. (2021) Design of a new effector recognition specificity in a plant NLR immune receptor by molecular engineering of its integrated decoy domain. bioRxiv 2021.04.24.441256; doi: doi: 10.1101/2021.04.24.441256
Chaiharn M, Theantana T, Pathom-aree W (2020) Evaluation of Biocontrol Activities of Streptomyces spp. against Rice Blast Disease Fungi. Pathogens 9(2): 126. doi: 10.3390/pathogens9020126
Chakraborty A, Hussain A, Sabnam N (2023) Uncovering the structural stability of Magnaporthe oryzae effectors: a secretome-wide in silico analysis. J Biomol Struct Dyn.: 1-22. doi: 10.1080/07391102.2023.2292795
, , , et al (2021) Endocytic protein Pal1 regulates appressorium formation and is required for full virulence of Magnaporthe oryzae. Molecular Plant Pathology 00: 1– 15. doi: 10.1111/mpp.13149
Chen X, Duan Y, Qiao F, et al. (2022) A secreted fungal effector suppresses rice immunity through host histone hypoacetylation. New Phytol. doi: 10.1111/nph.18265
Chen Y, Wu X, Chen C, et al. (2022) Proteomics Analysis Reveals the Molecular Mechanism of MoPer1 Regulating the Development and Pathogenicity of Magnaporthe oryzae. Front Cell Infect Microbiol. 12: 926771. doi: 10.3389/fcimb.2022.926771
, , , et al. (2023) Two nucleotide sugar transporters are important for cell wall integrity and full virulence of Magnaporthe oryzae. Molecular Plant Pathology 24: 374– 390. doi: 10.1111/mpp.13304
Chen P, Gao G, Lou G, et al. (2023) Improvement of Rice Blast Resistance in TGMS Line HD9802S through Optimized Anther Culture and Molecular Marker-Assisted Selection. Int J Mol Sci. 24(19): 14446. doi: 10.3390/ijms241914446
Chung H, Goh J, Han SS, et al. (2020) Comparative Pathogenicity and Host Ranges of Magnaporthe oryzae and Related Species. Plant Pathol J. 36(4): 305-313. doi: 10.5423/PPJ.FT.04.2020.0068
Chuwa CJ, Mabagala RB, Reuben MSOW (2015) Pathogenic Variation and Molecular Characterization of Pyricularia oryzae, Causal Agent of Rice Blast Disease in Tanzania. International Journal of Science and Research (IJSR) 4(11): 1131-1139. ISSN (Online): 2319-7064
Consolo VF, Milazzo J, Adreit H, Asselborn M, Liberman C, Maumary R, Bonell L, Tharreau D, Pedraza MV (2017) Caracterización de cepas de «Magnaporthe oryzae» de distintos hospedantes y virulencia en arroz y trigo. E.E.A. Concepción del Uruguay, C.R. Entre Ríos
Couch BC, Kohn LM (2002) A multilocus gene genealogy concordant with host preference indicates segregation of a new species, Magnaporthe oryzae from M. grisea. Mycologia 94: 683-693. doi: 10.1080/15572536.2003.11833196
Cruz MFA, Rios JA, Araujo L, et al. (2016) Infection process of Pyricularia oryzae on the leaves of wheat seedlings. Tropical Plant Pathology 41(2): 123–127. doi: 10.1007/s40858-016-0068-6
Cruz-Mireles N, Eseola AB, Osés-Ruiz M, et al. (2021) From appressorium to transpressorium—Defining the morphogenetic basis of host cell invasion by the rice blast fungus. PLoS Pathog 17(7): e1009779. doi: 10.1371/journal.ppat.1009779
Cúndom MA, Gutiérrez SA, Miño R, Duarte J (2008) Prevalencia e incidencia de las enfermedades del tallo y vainas foliares del arroz en la provincia de Corrientes. 1º Congreso de Fitopatología. Libro de Resúmenes: 169. Córdoba. 28-30 de mayo. p. 382. ISBN/ISSN: 978-987-24373-0-51.
D’Ávila LS, Corsi De Filippi MC, Café-Filho AC (2022) Fungicide resistance in Pyricularia oryzae populations from southern and northern Brazil and evidence of fitness costs for QoI-resistant isolates. Crop Protection 153: 105887. doi: 10.1016/j.cropro.2021.105887
De la Concepcion JC, Franceschetti M, Maqbool A, et al. (2018) Polymorphic residues in rice NLRs expand binding and response to effectors of the blast pathogen. Nature Plantsvolume 4: 576–585. doi: 10.1038/s41477-018-0194-x
De la Concepcion JC, Fujisaki K, Bentham AR, et al. (2022) A blast fungus zinc-finger fold effector binds to a hydrophobic pocket in host Exo70 proteins to modulate immune recognition in rice. Proc Natl Acad Sci U S A. 119(43): e2210559119. doi: 10.1073/pnas.221055911
, , , et al. (2023) Zinc-finger (ZiF) fold secreted effectors form a functionally diverse family across lineages of the blast fungus Magnaporthe oryzae.
, , , et al. (2023) Genetic diversity of an effector gene, AvrPi9, of rice blast pathogen in Thailand and characterization of its promoter. Plant Pathology 00: 1–13. doi: 10.1111/ppa.13798
Dean RA, Talbot NJ, Ebbole DJ, et al. (2005) The genome sequence of the rice blast fungus Magnaporthe grisea. Nature 434(7036): 980‐986. doi: 10.1038/nature03449
Debona D, Rios JA, Nascimento KJ, et al. (2016) Influence of magnesium on physiological responses of wheat infected by Pyricularia oryzae. Plant Pathology 65: 114-123. doi: 10.1111/ppa.12390
Deng Y, Zhai K, Xie Z, et al. (2017) Epigenetic regulation of antagonistic receptors confers rice blast resistance with yield balance. Science 355: 962-965. doi: 10.1126/science.aai8898
Deng S, Xu L, Xu Z, et al. (2021) A putative PKA phosphorylation site S227 in MoSom1 is essential for infection-related morphogenesis and pathogenicity in Magnaporthe oryzae. Cell Microbiol.: e13370. doi: 10.1111/cmi.13370
Ding Y, Yuan J, Mo F, et al. (2023) A pH-Responsive Essential Oil Delivery System Based on Metal-organic Framework (ZIF-8) for Preventing Fungal Disease. J Agric Food Chem. doi: 10.1021/acs.jafc.3c04299
Dirchwolf PM, Gutiérrez SA, Carmona MA (2015) Resultados preliminares en el control del tizón del arroz. (Pyricularia oryzae). Libro de resúmenes. XV Jornadas Fitosanitarias.
Dirchwolf PM, Gutiérrez SA, Carmona MA (2015) Incidencia del tizón (Pyricularia oryzae) en cultivos de arroz. Libro de resúmenes. XV Jornadas Fitosanitarias.
Dirchwolf PM (2015) Monitoreo e identificación de las enfermedades del cultivo de arroz. Campaña 2014/15. Faculta de Ciencias Agrarias. Universidad Nacional del Nordeste.
Dong Y, Li Y, Zhao M, et al. (2015) Global Genome and Transcriptome Analyses of Magnaporthe oryzae Epidemic Isolate 98-06 Uncover Novel Effectors and Pathogenicity-Related Genes, Revealing Gene Gain and Lose Dynamics in Genome Evolution. PLoS Pathog 11(4): e1004801. doi: 10.1371/journal.ppat.1004801
Duan G, Bao J, Chen X, Xie J, Liu Y, Chen H, Zheng H, Tang W, Wang Z (2021) Large-Scale Genome Scanning within Exonic Regions Revealed the Contributions of Selective Sweep Prone Genes to Host Divergence and Adaptation in Magnaporthe oryzae Species Complex. Microorganisms 9(3): 562. doi: 10.3390/microorganisms9030562
Dulal N, Wilson RA (2024) Paths of Least Resistance: Unconventional Effector Secretion by Fungal and Oomycete Plant Pathogens. Mol Plant Microbe Interact. 37(9): 653-661. doi: 10.1094/MPMI-12-23-0212-CR
Escolà G, González-Miguel VM, Campo S, et al. (2023) Development and Genome-Wide Analysis of a Blast-Resistant japonica Rice Variety. Plants (Basel) 12(20): 3536. doi: 10.3390/plants12203536
Eseola AB, Ryder LS, Osés-Ruiz M, et al. (2021) Investigating the cell and developmental biology of plant infection by the rice blast fungus Magnaporthe oryzae. Fungal Genetics and Biology 154: 103562. doi: 10.1016/j.fgb.2021.103562
Esquivel BD and White TC (2017) Accumulation of Azole Drugs in the Fungal Plant Pathogen Magnaporthe oryzae Is the Result of Facilitated Diffusion Influx. Front. Microbiol. 8: 1320. doi: 10.3389/fmicb.2017.01320
Fang H, Zhang F, Zhang C, et al. (2022) Function of hydroxycinnamoyl transferases for the biosynthesis of phenolamides in rice resistance to Magnaporthe oryzae. J Genet Genomics: S1673-8527(22)00048-0. doi: 10.1016/j.jgg.2022.02.008
Feng W, Yin Z, Wu H, et al. (2021) Balancing of the mitotic exit network and cell wall integrity signaling governs the development and pathogenicity in Magnaporthe oryzae. PLoS Pathog 17(1): e1009080. doi: 10.1371/journal.ppat.1009080
Feng Q, Wang H, Yang X-M, et al. (2022) Osa-miR160a confers broad-spectrum resistance to fungal and bacterial pathogens in rice. New Phytol. doi: 10.1111/nph.18491
Fernandez J, Orth K (2018) Rise of a Cereal Killer: The Biology of Magnaporthe oryzae Biotrophic Growth. Trends in Microbiology (In press). doi: 10.1016/j.tim.2017.12.007
Fernandez J (2023) The Phantom Menace: latest findings on effector biology in the rice blast fungus. aBIOTECH 4(2):140-154. doi: 10.1007/s42994-023-00099-4
Gallet R, Fontaine C, Bonnot F, Milazzo J, Tertois C, Adreit H, Ravigné V, Fournier E, Tharreau D (2016) Evolution of Compatibility Range in the Rice−Magnaporthe oryzae System: An Uneven Distribution of R Genes Between Rice Subspecies. Phytopathology 106(4): 348-354. doi: 10.1094/PHYTO-07-15-0169-R
Gao M, He Y, Yin X, et al. (2021) Ca2+ sensor-mediated ROS scavenging suppresses rice immunity and is exploited by a fungal effector. Cell 184: 5391-5404.e17. doi: 10.1016/j.cell.2021.09.009
Garcés Fiallos FR, Díaz Coronel TG, Aguirre Calderón AJ (2012) Severidad de la quemazón (Pyricularia oryzae cav.) en germoplasma de arroz F1 en la zona central del litoral ecuatoriano. Ciencia y Tecnología 5(2): 1-6. ISSN-e 1390-4043
Gashaw G, Alemu T, Tesfaye K (2014) Morphological, physiological and biochemical studies on Pyricularia grisea isolates causing blast disease on finger millet in Ethiopia. Journal of Applied Biosciences 74: 6059-6071. doi: 10.4314/jab.v74i1.2
Giraldo MC, Dagdas YF, Gupta YK, et al. (2013) Two distinct secretion systems facilitate tissue invasion by the rice blast fungus Magnaporthe oryzae. Nature Communications 4, Article number: 1996. doi: 10.1038/ncomms2996
Gladieux P, Condon B, Ravel S, et al. (2018) Gene flow between divergent cereal- and grass-specific lineages of the rice blast fungus Magnaporthe oryzae. mBio 9:e01219-17. doi: 10.1128/mBio.01219-17
Gong Z, Ning N, Li Z, et al. (2022) Two Magnaporthe appressoria-specific (MAS) proteins, MoMas3 and MoMas5, are required for suppressing host innate immunity and promoting biotrophic growth in rice cells. Mol Plant Pathol. doi: 10.1111/mpp.13226
Guan L, Wang H, Chen J, et al. (2023) Isolation and Identification of Culturable Bacteria from South China Seawater and Preliminary Screening of Marine Biocontrol Bacteria. Microorganisms 11(12): 2933. doi: 10.3390/microorganisms11122933
Guo H, Du Q, Xie Y, et al. (2021) Identification of Rice Blast Loss-of-Function Mutant Alleles in the Wheat Genome as a New Strategy for Wheat Blast Resistance Breeding. Front Genet. 12: 623419. doi: 10.3389/fgene.2021.623419
Guo Z, Liu X, Wang N, et al. (2022) Membrane component ergosterol builds a platform for promoting effector secretion and virulence in Magnaporthe oryzae. New Phytol. doi: 10.1111/nph.18575
Gupta L, Vermani M, Kaur Ahluwalia S, Vijayaraghavan P (2021) Molecular virulence determinants of Magnaporthe oryzae: disease pathogenesis and recent interventions for disease management in rice plant. Mycology 12(3): 174-187. doi: 10.1080/21501203.2020.1868594
Gutiérrez SA, Cúndom MA (2015) Pyricularia oryzae en cultivos de cebada en Corrientes (Argentina). Summa Phytopathologica 41(4): 318-320. doi: 10.1590/0100-5405/2063
Hao Z, Tian J, Fang H, et al. (2022) A VQ-motif-containing protein fine-tunes rice immunity and growth by a hierarchical regulatory mechanism. Cell Rep. 40(7): 111235. doi: 10.1016/j.celrep.2022.111235
Hernández-RodríguezI A, Rives-RodríguezII N, Acebo-GuerreroI Y, et al. (2014) Potencialidades de las bacterias diazotróficas asociativas en la promoción del crecimiento vegetal y el control de Pyricularia oryzae (Sacc.) en el cultivo del arroz (Oryza sativa L.). Rev. Protección Veg. 29(1): 1-10. ISSN 1010-2752
Howard RJ, Valent B (1996) Breaking and entering: host penetration by the fungal rice blast pathogen. Annual Review of Microbiology 50: 491-512. doi: 10.1146/annurev.micro.50.1.491
Hu J, Liu M, Zhang A, et al. (2022) Co-evolved ascorbate oxidases of plant and the blast fungus orchestrate host apoplast redox state to modulate rice immunity. Mol Plant. S1674-2052(22)00221-0. doi: 10.1016/j.molp.2022.07.001
Hu Q, Wu Y, Hong T, et al. (2023) OsMED16, a tail subunit of Mediator complex, interacts with OsE2Fa to synergistically regulate rice leaf development and blast resistance. Int J Biol Macromol. 253(Pt 2): 126728. doi: 10.1016/j.ijbiomac.2023.126728
Huang W, Liu X, Zhou X, et al. (2020) Calcium Signaling Is Suppressed in Magnaporthe oryzae Conidia by Bacillus cereus HS24. Phytopathology 110(2): 309-316. doi: 10.1094/PHYTO-08-18-0311-R
Huang C, Li L, Wang L, et al. (2022) The Amino Acid Permease MoGap1 Regulates TOR Activity and Autophagy in Magnaporthe oryzae. Int J Mol Sci. 23(21): 13663. doi: 10.3390/ijms232113663
, , , et al. (2023) The triglyceride catabolism regulated by a serine/threonine protein phosphatase, Smek1, is required for development and plant infection in Magnaporthe oryzae. Molecular Plant Pathology 24: 1256–1272. doi: 10.1111/mpp.13368
IslamT, Ansary MWR, Rahman MM (2023) Magnaporthe oryzae and Its Pathotypes: A Potential Plant Pandemic Threat to Global Food Security. In: Scott, B., Mesarich, C. (eds) Plant Relationships. The Mycota, vol 5. Springer, Cham. doi: 10.1007/978-3-031-16503-0_18
Jalilian A, Bagheri A, Chalvon V, et al. (2023) The RLCK subfamily VII-4 controls pattern-triggered immunity and basal resistance to bacterial and fungal pathogens in rice. Plant J. doi: 10.1111/tpj.16323
Jarosch B, Collins NC, Zellerhoff N, Schaffrath U (2005) RAR1, ROR1, and the Actin Cytoskeleton Contribute to Basal Resistance to Magnaporthe grisea in Barley. Molecular Plant-Microbe Interactions 18(5): 397-404. doi: 10.1094/MPMI-18-0397
Javed M, Reddy B, Sheoran N, et al. (2023) Unraveling the Transcriptional Network Regulated by miRNAs in Blast-Resistant and Blast. Gene:147718. doi: 10.1016/j.gene.2023.147718
Jia Y, McAdams SA, Bryan GT, Hershey HP, Valent B (2000) Direct interaction of resistance gene and avirulence gene product confers rice blast resistance. EMBO Journal 19: 4004-4014. doi: 10.1093/emboj/19.15.4004
Jiang Z, Shi D, Chen Y, et al. (2023) Discovery of novel isopropanolamine inhibitors against MoTPS1 as potential fungicides with unique mechanisms. Eur J Med Chem. 260: 115755. doi: 10.1016/j.ejmech.2023.115755
Kapoor R, Kumar G, Pawar L, et al. (2022) Stress responsive OsHyPRP16 promoter driven early expression of resistance gene Pi54 potentiate the resistance against Magnaporthe oryzae in transgenic rice. Plant Sci. 10: 111413. doi: 10.1016/j.plantsci.2022.111413
Kaundal R, Kapoor AS, Raghava GP (2006) Machine learning techniques in disease forecasting: a case study on rice blast prediction. BMC Bioinformatics 7: 485. doi: 10.1186/1471-2105-7-485
Kawasaki-Tanaka A, Fukuta Y (2014) Genetic variation in resistance to blast disease (Pyricularia oryzae Cavara) in Japanese rice (Oryza sativa L.), as determined using a differential system. Breeding Science 64(2): 183-192. doi: 10.1270/jsbbs.64.183
Kawasaki-Tanaka A, Hayashi N, Yanagihara S, Fukuta Y (2016) Diversity and Distribution of Rice Blast (Pyricularia oryzae Cavara) Races in Japan. Plant Disease 100(4): 816-823. doi: 10.1094/PDIS-04-15-0442-RE
Kgosi VT, Tingting B, Ying Z, Liu H (2022) Anti-Fungal Analysis of Bacillus subtilis DL76 on Conidiation, Appressorium Formation, Growth, Multiple Stress Response, and Pathogenicity in Magnaporthe oryzae. International Journal of Molecular Sciences 23(10): 5314. doi: 10.3390/ijms23105314
Kim YS, Dixon EW, Vincelli P, Farman ML (2003) Field Resistance to Strobilurin (Q(o)I) Fungicides in Pyricularia grisea Caused by Mutations in the Mitochondrial Cytochrome b Gene. Phytopathology 93(7): 891-900. doi: 10.1094/PHYTO.2003.93.7.891
Kim CY, Park JY, Choi G, et al. (2021) A rice gene encoding glycosyl hydrolase plays contrasting roles in immunity depending on the type of pathogens. Mol Plant Pathol. doi: 10.1111/mpp.13167
Kim KT, Ko J, Song H, et al. (2019) Evolution of the Genes Encoding Effector Candidates Within Multiple Pathotypes of Magnaporthe oryzae. Front Microbiol. 10: 2575. doi: 10.3389/fmicb.2019.02575
Kim M, Lee SH, Jeon J (2023) A Nucleolar Protein, MoRRP8 Is Required for Development and Pathogenicity in the Rice Blast Fungus. Mycobiology 51(5): 273-280. doi: 10.1080/12298093.2023.2257996
Klaubauf S, Tharreau D, Fournier E, et al. (2014) Resolving the polyphyletic nature of Pyricularia (Pyriculariaceae). Studies in Mycology 79: 85–120. doi: 10.1016/j.simyco.2014.09.004
Kumar V, Jain P, Venkadesan S, et al. (2021) Understanding Rice-Magnaporthe Oryzae Interaction in Resistant and Susceptible Cultivars of Rice under Panicle Blast Infection Using a Time-Course Transcriptome Analysis. Genes (Basel) 12(2): 301. doi: 10.3390/genes12020301
Kumar V, Koul B, Taak P, et al. (2023) Journey of Trichoderma from Pilot Scale to Mass Production: A Review. Agriculture 13(10): 2022. doi: 10.3390/agriculture13102022
Kunova A, Pizzatti C, Cortesi P (2013) Impact of tricyclazole and azoxystrobin on growth, sporulation and secondary infection of the rice blast fungus, Magnaporthe oryzae. Pest Management Science 69: 278-284. doi: 10.1002/ps.3386
Kunova A, Pizzatti C, Bonaldi M, Cortesi P (2014) Sensitivity of nonexposed and exposed populations of Magnaporthe oryzae from rice to tricyclazole and azoxystrobin. Plant Disease 98: 512-518. doi: 10.1094/PDIS-04-13-0432-RE
Kurahashi Y (2001 ) Melanin biosynthesis inhibitors (MBIs) for control of rice blast. Pesticide Outlook 12: 32-35. doi: 10.1039/B100806O
Ladera-Carmona M, Zheng Y, Moorlach B, et al. (2024) Exogenous dsRNA-Mediated Control of Rice Blast Fungus Involves Sequence-Specific RNAi and Sequence-Unspecific Fungal Stress Responses. PREPRINT (Version 1) available at Research Square. doi: 10.21203/rs.3.rs-4538504/v1
Lahari Z, van Boerdonk S, Omoboye OO, et al. (2023) Strigolactone deficiency induces jasmonate, sugar and flavonoid phytoalexin accumulation enhancing rice defense against the blast fungus Pyricularia oryzae. New Phytol. doi: 10.1111/nph.19354
Lamanchai K, Smirnoff N, Salmon DL, et al. (2022) OsVTC1-1 Gene Silencing Promotes a Defense Response in Rice and Enhances Resistance to Magnaporthe oryzae. Plants (Basel) 11(17): 2189. doi: 10.3390/plants11172189
Lambou K, Tag A, Lassagne A, et al. (2024) The bZIP transcription factor BIP1 of the rice blast fungus is essential for infection and regulates a specific set of appressorium genes. PLoS Pathog. 20(1): e1011945. doi: 10.1371/journal.ppat.1011945
Langner T, Białas A, Kamoun S (2018) The blast fungus decoded: genomes in flux. mBio 9:e00571-18. doi: 10.1128/mBio.00571-18
Langner T, Harant A, Gomez-Luciano LB, et al. (2021) Genomic rearrangements generate hypervariable mini-chromosomes in host-specific isolates of the blast fungus. PLoS Genet 17(2): e1009386. doi: 10.1371/journal.pgen.1009386
Lee SH, Oh YT, Lee DY, et al. (2022) Large-Scale Screening of the Plant Extracts for Antifungal Activity against the Plant Pathogenic Fungi. Plant Pathol J. 38(6): 685-691. doi: 10.5423/PPJ.NT.07.2022.0098
Lee GH, Min CW, Jang JW, et al. (2023) Analysis of post-translational modification dynamics unveiled novel insights into Rice responses to MSP1. J Proteomics. 287: 104970. doi: 10.1016/j.jprot.2023.104970
Lee GH, Min CW, Jang JW, et al. (2023) Dataset on post-translational modifications proteome analysis of MSP1-overexpressing rice leaf proteins. Data Brief. 50: 109573. doi: 10.1016/j.dib.2023.109573
Lei LY, Xiong ZX, Li JL, et al. (2023) Biological control of Magnaporthe oryzae using natively isolated Bacillus subtilis G5 from Oryza officinalis roots. Front Microbiol. 14: 1264000. doi: 10.3389/fmicb.2023.1264000
Li Y, Yue X, Que Y, et al. (2014) Characterisation of Four LIM Protein-Encoding Genes Involved in Infection-Related Development and Pathogenicity by the Rice Blast Fungus Magnaporthe oryzae. PLoS ONE 9(2): e88246. doi: 10.1371/journal.pone.0088246
Li W, Xiong Y, Lai LB, et al. (2021) The rice RNase P protein subunit Rpp30 confers broad-spectrum resistance to fungal and bacterial pathogens. Plant Biotechnol J. doi: 10.1111/pbi.13612
Li Y, Zheng X, Pei M, et al. (2021) Identification and characterization of 19 predicted Myb-family DNA binding proteins in Magnaporthe oryzae concerning growth, conidiation, and pathogenicity. bioRxiv 2021.12.28.474317; doi: 10.1101/2021.12.28.474317
Li T, Xu J, Gao H, et al. (2022) The G143A/S substitution of mitochondrially encoded cytochrome b (Cytb) in Magnaporthe oryzae confers resistance to quinone outside inhibitors. Pest Manag Sci. 78(11): 4850-4858. doi: 10.1002/ps.7106
Li R, Bi R, Cai H, et al. (2022) Melatonin Functions as a Broad-spectrum Antifungal by Targeting a Conserved Pathogen Protein Kinase. J Pineal Res. doi: 10.1111/jpi.12839
Li G, Gong Z, Dulal N, et al. (2023) A protein kinase coordinates cycles of autophagy and glutaminolysis in invasive hyphae of the fungus Magnaporthe oryzae within rice cells. Nat Commun. 14(1): 4146. doi: 10.1038/s41467-023-39880-w
Li Z, Yang K, Ye W, et al. (2024) Rice leaf disease detection based on enhanced feature fusion and target adaptation. Plant Pathology 00: 1–13. doi: 10.1111/ppa.13866
Li D, Li T, Yang X, et al. (2024) Carbon nanosol promotes plant growth and broad-spectrum resistance. Environ Res.: 118635. doi: 10.1016/j.envres.2024.118635
Liang D, Yu J, Song T, et al. (2022) Genome-Wide Prediction and Analysis of Oryza Species NRP Genes in Rice Blast Resistance. Int J Mol Sci. 23(19): 11967. doi: 10.3390/ijms231911967
Lindsay RJ, Kershaw MJ, Pawlowska BJ, et al. (2016) Harbouring public good mutants within a pathogen population can increase both fitness and virulence. eLife 2016;5:e18678. doi: 10.7554/eLife.18678
Liu Y, Pagac M, Yang F, Patkar RN, Naqvi NI (2021) Fungal Jasmonate as a Novel Morphogenetic Signal for Pathogenesis. J Fungi (Basel) 7(9): 693. doi: 10.3390/jof7090693
Liu C, Liu T, Lv Z, et al. (2022) A Calcineurin Regulator MoRCN1 Is Important for Asexual Development, Stress Response, and Plant Infection of Magnaporthe oryzae. Front Plant Sci. 13: 925645. doi: 10.3389/fpls.2022.925645
Liu W, Wang J, Zhang H, et al. (2022) Transcriptome analysis of the production enhancement mechanism of antimicrobial lipopeptides of Streptomyces bikiniensis HD-087 by co-culture with Magnaporthe oryzae Guy11. Microb Cell Fact. 21(1): 187. doi: 10.1186/s12934-022-01913-2
Liu M, Wang F, He B, et al. (2024) Targeting Magnaporthe oryzae effector MoErs1 and host papain-like protease OsRD21 interaction to combat rice blast. Nat Plants. doi: 10.1038/s41477-024-01642-x
Long DH, Lee FN, TeBeest DO (2000) Effect of nitrogen fertilizer on disease progress on susceptible and resistant cultivars. Plant Disease 84: 403-409. doi: 10.1094/PDIS.2000.84.4.403
Long DH, Correll JC, Lee FN, TeBeest DO (2001) Rice blast epidemics initiated by infested rice grain on the soil surface. Plant Disease 85: 612-616. doi: 10.1094/PDIS.2001.85.6.612
Ioos R (2022) Molecular Detection of Wheat Blast Pathogen in Seeds. Methods Mol Biol. 2536: 139-153. doi: 10.1007/978-1-0716-2517-0_9
Lu L, Wang Q, Jia Y, et al. (2018) Selection and mutation of the avirulence gene AVR‐Pii of the rice blast fungus Magnaporthe oryzae. Plant Pathology. doi: 10.1111/ppa.12935
Lu K, Chen R, Yang Y, et al. (2023) Involvement of the Cell Wall-Integrity Pathway in Signal Recognition, Cell-Wall Biosynthesis, and Virulence in Magnaporthe oryzae. Mol Plant Microbe Interact. 36(10): 608-622. doi: 10.1094/MPMI-11-22-0231-CR
Luo J, Zhang N (2021) The Rice Blast Fungus and Allied Species: A Monograph of the Fungal Order Magnaporthales. LINK
Luo X, Wang L, Fu Y, et al. (2022) FERONIA-like receptor 1-mediated calcium ion homeostasis is involved in the immune response. Front Plant Sci. 13: 934195. doi: 10.3389/fpls.2022.934195
, , (2023) Comparative omics analysis for novel target discovery in plant pathogens: A case study for Magnaporthe oryzae. Plant Pathology 00: 1–14. doi: 10.1111/ppa.13840
Lv W, Xiao Y, Xu Z, et al. (2022) The Paxillin MoPax1 Activates Mitogen-Activated Protein (MAP) Kinase Signaling Pathways and Autophagy through MAP Kinase Activator MoMka1 during Appressorium-Mediated Plant Infection by the Rice Blast Fungus Magnaporthe oryzae. mBio:e0221822. doi: 10.1128/mbio.02218-22
Ma X, Duan G, Chen H, et al. (2022) Characterization of infected process and primary mechanism in rice Acuce defense against rice blast fungus, Magnaporthe oryzae. Plant Mol Biol. doi: 10.1007/s11103-022-01296-3
Ma Y, Yang J, Dong J, et al. (2022) Overexpression of OsGF14f Enhances Quantitative Leaf Blast and Bacterial Blight Resistance in Rice. Int J Mol Sci. 23(13): 7440. doi: 10.3390/ijms23137440
Ma L, Yu Y, Li C, et al. (2022) Genome-Wide Association Study Identifies a Rice Panicle Blast Resistance Gene Pb3 Encoding NLR Protein. Int J Mol Sci. 23(22): 14032. doi: 10.3390/ijms232214032
Maidment JHR, Franceschetti M, Maqbool A, et al. (2021) Multiple variants of the fungal effector AVR-Pik bind the HMA domain of the rice protein OsHIPP19, providing a foundation to engineer plant defence. J Biol Chem.: 100371. doi: 10.1016/j.jbc.2021.100371
Maina AW, Oerke EC (2024) Hyperspectral imaging for quantifying Magnaporthe oryzae sporulation on rice genotypes. Plant Methods 20: 87. doi: 10.1186/s13007-024-01215-1
Martinez-D’Alto A, Yan X, Detomasi TC, et al. (2023) Characterization of a unique polysaccharide monooxygenase from the plant pathogen Magnaporthe oryzae. Proc Natl Acad Sci U S A. 120(8): e2215426120. doi: 10.1073/pnas.2215426120
Masaki HI, de Villiers S, Qi P, e al. (2023) Non-host resistance to the finger millet blast pathogen Magnaporthe oryzae is modulated by a resistance response to PWL1 and PWL2 in Eragrostis curvula but not in related Chloridoid species. Mol Plant Microbe Interact. doi: 10.1094/MPMI-01-23-0012-R
Masaki HI, de Villiers S, Qi P, et al. (2023) Host Specificity Controlled by PWL1 and PWL2 Effector Genes in the Finger Millet Blast Pathogen Magnaporthe oryzae in Eastern Africa. Mol Plant Microbe Interact. 36(9): 584-591. doi: 10.1094/MPMI-01-23-0012-R
Miao J, Zhao G, Wang B, et al. (2020) Three point‐mutations in cytochrome b confer resistance to trifloxystrobin in Magnaporthe oryzae. Pest Manag Sci 76: 4258-4267. doi: 10.1002/ps.5990
Mmbando GS (2024) The link between changing in host carbon allocation and resistance to Magnaporthe oryzae: a possible tactic for mitigating the rice blast fungus. Plant Signal Behav. 19(1): 2326870. doi: 10.1080/15592324.2024.2326870
Mohapatra NK, Mukherjee AK, Suriya Rao AV, Nayak P (2008) Disease progress curves in the rice blast pathosystem compared with the Logistic and Gompertz models. ARPN Journal of Agricultural and Biological Science 3(1): 28-37. ISSN 1990-6145
Moreira SI, Ceresini PC, Alves E (2015) Reprodução Sexuada em Pyricularia oryzae. Summa Phytopathologica 41(3): 75-182. doi: 10.1590/0100-5405/2067
Motoyama T (2020) Secondary Metabolites of the Rice Blast Fungus Pyricularia oryzae: Biosynthesis and Biological Function. Int J Mol Sci. 21(22): 8698. doi: 10.3390/ijms21228698
Motoyama T, Kondoh Y, Shimizu T, et al. (2022) Identification of Scytalone Dehydratase Inhibitors Effective against Melanin Biosynthesis Dehydratase Inhibitor-Resistant Pyricularia oryzae. J. Agric. Food Chem.70: 3109–3116. doi: 10.1021/acs.jafc.1c04984
Mutiga SK, Rotich F, Were VM, et al. (2021) Integrated strategies for durable rice blast resistance in sub-Saharan Africa. Plant Disease. doi: 10.1094/PDIS-03-21-0593-FE
Mutiga SK, Orwa P, Nganga EM, et al. (2023) Characterization of Blast Resistance in a Diverse Rice Panel from Sub-Saharan Africa. Phytopathology 113(7): 1278-1288. doi: 10.1094/PHYTO-10-22-0379-R
Nakamoto AA, Joubert PM, Krasileva KV (2023) Intraspecific variation of transposable elements reveals differences in the evolutionary history of fungal phytopathogen pathotypes. Genome Biol Evol.: evad206. doi: 10.1093/gbe/evad206
Nguyen MV, Han JW, Kim H, Choi GJ (2022) Curvicollide D, a new modified γ-lactone from the culture broth of Albifimbria verrucaria and its antifungal activity against plant pathogenic fungi. J Antibiot (Tokyo). doi: 10.1038/s41429-022-00541-7
Ninkuu V, Yan J, Zhang L, et al. (2022) Hrip1 mediates rice cell wall fortification and phytoalexins elicitation to confer immunity against Magnaporthe oryzae. Front Plant Sci. 13: 980821. doi: 10.3389/fpls.2022.980821
Odjo T, Kawasaki-Tanaka A, Noda T, et al. (2014) Pathogenicity analysis of Blast (Pyricularia oryzae Cavara) isolates from west Africa. JARQ 48(4): 403-412.
Odjo T, Diagne D, Adreit H, et al. (2021) Structure of African populations of Pyricularia oryzae from rice. Phytopathology. doi: 10.1094/PHYTO-05-20-0186-R
Oliveira SC, Castroagudín VL, Maciel JLN, et al. (2015) Resistência cruzada aos fungicidas IQo azoxistrobina e piraclostrobina no patógeno da brusone do trigo Pyricularia oryzae no Brasil. Summa Phytopathologica 41(4): 298-304. doi: 10.1590/0100-5405/2072
Oliveira-Garcia E, Budot BO, Manangkil J, et al. (2023) An efficient method for screening rice breeding lines against races of Magnaporthe oryzae. Plant Dis. doi: 10.1094/PDIS-05-23-0922-RE
Oliveira-Garcia E, Yan X, Oses-Ruiz M, et al. (2023) Effector-triggered susceptibility by the rice blast fungus Magnaporthe oryzae. New Phytol. doi: 10.1111/nph.19446
Orbach MJ, Farrall L, Sweigard JA, et al. (2000) A telomeric avirulence gene determines efficacy for the rice blast gene Pi-ta. Plant Cell 12: 2019-2032. doi: 10.1105/tpc.12.11.2019
Osés-Ruiz M, Cruz-Mireles N, Martin-Urdiroz M, et al. (2021) Appressorium-mediated plant infection by Magnaporthe oryzae is regulated by a Pmk1-dependent hierarchical transcriptional network. Nat Microbiol 6: 1383–1397. doi: 10.1038/s41564-021-00978-w
Pagliaccia D, Urak RZ, Wong F, et al. (2018) Genetic Structure of the Rice Blast Pathogen (Magnaporthe oryzae) over a Decade in North Central California Rice Fields. Microb Ecol. 75(2): 310–317. doi: 10.1007/s00248-017-1029-4
Palanna KB, Vinaykumar HD, Prasanna SK, et al. (2023) Exploring the diversity of virulence genes in the Magnaporthe population infecting millets and rice in India. Front Plant Sci. 14: 1131315. doi: 10.3389/fpls.2023.1131315
, , , et al. (2022) Intracellular Ca2+ accumulation triggered by caffeine provokes resistance against a broad range of biotic stress in rice. Plant, Cell & Environment, 1– 16. doi: 10.1111/pce.14273
Pedraza MV, Liberman CA, Nuñez Bordoy E, Asselborn MN (2014) Hospedantes secundarios como fuente de inóculo potencial del quemado del arroz en Argentina. Editor/es: 3° Congreso Argentino de Fitopatología. Libro de Resúmenes. 3° Congreso Argentino de Fitopatologia. Página/s: 281.
Peng Z, Li L, Wu S, et al. (2021) Frequencies and Variations of Magnaporthe oryzae Avirulence Genes in Hunan Province, China. Plant Disease. doi: 10.1094/PDIS-01-21-0008-RE
Raveloson H, Ratsimiala Ramonta I, et al. (2018) Long-term survival of blast pathogen in infected rice residues as major source of primary inoculum in high altitude upland ecology. Plant Pathol 67: 610-618. doi: 10.1111/ppa.12790
Rahnama M, Condon B, Ascari JP, et al. (2021) Recombination of standing variation in a multi-hybrid swarm drove adaptive radiation in a fungal pathogen and gave rise to two pandemic plant diseases. bioRxiv 2021.11.24.469688; doi: 10.1101/2021.11.24.469688
Ren Z, Dong X, Guan L, et al. (2024) Sirt5-mediated lysine desuccinylation regulates oxidative stress adaptation in Magnaporthe oryzae during host intracellular infection. New Phytol. doi: 10.1111/nph.19683
Rios JA, Rodrigues FA, Debona D, et al. (2014). Induction of resistance to Pyricularia oryzae in wheat by acibenzolar-S-methyl, ethylene and jasmonic acid. Tropical Plant Pathology 39(3): 224-233. doi: 10.1590/S1982-56762014000300006
Rossman AY, Howard R, Valent B (1990) Pyricularia grisea, the correct name for the rice blast disease fungus. Mycologia 82: 509-512.
Ryder LS, Lopez SG, Michels L, et al. (2023) A molecular mechanosensor for real-time visualization of appressorium membrane tension in Magnaporthe oryzae. Nat Microbiol. 8(8): 1508-1519. doi: 10.1038/s41564-023-01430-x
Sabnam N, Roy Barman S (2017) WISH, a novel CFEM GPCR is indispensable for surface sensing, asexual and pathogenic differentiation in rice blast fungus. Fungal Genetincs and Biology 105: 37-51. doi: 10.1016/j.fgb.2017.05.006
Sakulkoo W, Osés-Ruiz M, Oliveira Garcia E, et al. (2018) A single fungal MAP kinase controls plant cell-to-cell invasion by the rice blast fungus. Science 359, Issue 6382: 1399-1403. doi: 10.1126/science.aaq0892
Salman EK, Ghoniem KE, Badr ES, Emeran AA (2021) The Potential of Dimetindene Maleate Inducing Resistance through Activating Salicylic Acid Signaling Pathway in Rice Plants to Blast Fungus Magnaporthe oryzae. Pest Manag Sci. doi: 10.1002/ps.6673
Sarkar D, Majumder S, Giri K, Sabnam N (2022) In silico characterization, molecular docking, and dynamic simulation of a novel fungal cell-death suppressing effector, MoRlpA as potential cathepsin B-like cysteine protease inhibitor during rice blast infection. J Biomol Struct Dyn.: 1-18. doi: 10.1080/07391102.2022.2139763
Sarrocco S (2023) Biological Disease Control by Beneficial (Micro)Organisms: Selected Breakthroughs in the Past 50 Years. Phytopathology 113: 732-740. doi: 10.1094/PHYTO-11-22-0405-KD
Scheuermann KK, Vieira Raimondi J, Marschalek R, et al. (2012) Chapter 15 Magnaporthe oryzae Genetic Diversity and Its Outcomes on the Search for Durable Resistance. In: Caliskan M (Ed) Agricultural and Biological Sciences » «The Molecular Basis of Plant Genetic Diversity». doi: 10.5772/33479
Seong K, Krasileva KV (2021) Computational structural genomics unravels common folds and predicted functions in the secretome of fungal phytopathogen Magnaporthe oryzae. bioRxiv 2021.01.25.427855; doi: 10.1101/2021.01.25.427855
Sha Y, Zeng Q, Sui S (2020) Screening and Application of Bacillus Strains Isolated from Nonrhizospheric Rice Soil for the Biocontrol of Rice Blast. Plant Pathol J. 36(3): 231-243. doi: 10.5423/PPJ.OA.02.2020.0028
Shabbir A, Batool W, Yu D, et al. (2022) Magnaporthe oryzae Chloroplast Targeting Endo-β-1,4-Xylanase I MoXYL1A Regulates Conidiation, Appressorium Maturation and Virulence of the Rice Blast Fungus. Rice (N Y) 12;15(1): 44. doi: 10.1186/s12284-022-00584-2
Shen N, Han L, Liu Z, et al. (2024) The Microtubule End Binding Protein Mal3 Is Essential for the Dynamic Assembly of Microtubules during Magnaporthe oryzae Growth and Pathogenesis. Int J Mol Sci. 25(5): 2672. doi: 10.3390/ijms25052672
Shi NN, Ruan HC, Liu XZ, et al. (2018) Virulence structure of Magnaporthe oryzae populations from Fujian Province, China. Canadian Journal of Plant Pathology. doi: 10.1080/07060661.2018.1504821
, , , et al. (2021) MoWhi2 regulates appressorium formation and pathogenicity via the MoTor signalling pathway in Magnaporthe oryzae. Mol Plant Pathol. 00: 1– 15. doi: 10.1111/mpp.13074
Shi H, Xiong Q, Zhao Z, et al. (2023) Disruption of the Novel Small Protein RBR7 Leads to Enhanced Plant Resistance to Blast Disease. Rice (N Y) 16(1): 42. doi: 10.1186/s12284-023-00660-1
Shi H, Meng S, Qiu J, et al. (2024) MoAti1 mediates mitophagy by facilitating recruitment of MoAtg8 to promote invasive growth in Magnaporthe oryzae. Molecular Plant Pathology 25: e13439. doi: 10.1111/mpp.13439
Shimizu M, Hirabuchi A, Sugihara Y, et al. (2022) A genetically linked pair of NLR immune receptors shows contrasting patterns of evolution. Proc Natl Acad Sci U S A. Jul 5;119(27): e2116896119. doi: 10.1073/pnas.2116896119
Singh PK, Ray S, Thakur S, et al. (2018) Co-evolutionary interactions between host resistance and pathogen avirulence genes in rice-Magnaporthe oryzae pathosystem. Fungal Genetics and Biology 115: 9-19. doi: 10.1016/j.fgb.2018.04.005
Singh S, Sharma R, Nepolean T, et al. (2022) Identification of genes controlling compatible and incompatible reactions of pearl millet (Pennisetum glaucum) against blast (Magnaporthe grisea) pathogen through RNA-Seq. Front Plant Sci. 13: 981295. doi: 10.3389/fpls.2022.981295
Sirisathaworn T, Srirat T, Longya A, Jantasuriyarat C (2017) Evaluation of mating type distribution and genetic diversity of three Magnaporthe oryzae avirulence genes, PWL-2, AVR-Pii and Avr-Piz-t, in Thailand rice blast isolates. Agriculture and Natural Resources 51(1): 7-14. doi: 10.1016/j.anres.2016.08.005
Song L, Wang F, Liu C, et al. (2023) Isolation and Evaluation of Streptomyces melanogenes YBS22 with Potential Application for Biocontrol of Rice Blast Disease. Microorganisms 11(12): 2988. doi: 10.3390/microorganisms11122988
Srivastava D, Shamim M, Kumar D, et al. (2014) Characterization of Pyricularia Oryzae Causing Blast Disease in Rice (Oryza Sativa) from North India. International Journal of Scientific and Research Publications 4(7): 1-9. ISSN 2250-3153
Subba P, Saha P, Karthikkeyan G, et al. (2022) Metabolite profiling reveals overexpression of the global regulator, MoLAEA leads to increased synthesis of metabolites in Magnaporthe oryzae. J Appl Microbiol. doi: 10.1111/jam.15518
Sukanya SL, Yamini D, Fathima SK (2011) Eco-friendly management of Pyricularia oryzae – The causal agent of blast of paddy. Current Botany 2(8): 46-49. ISSN 2220-4822
Sun T, Jin X, Zhang X, et al. (2022) Design, Synthesis, and Biological Activity of Novel Laccase Inhibitors as Fungicides against Rice Blast. J Agric Food Chem. doi: 10.1021/acs.jafc.2c05144
, , , et al. (2024) Mutation of OsCDS5 confers broad-spectrum disease resistance in rice. Molecular Plant Pathology 25: e13430. doi: 10.1111/mpp.13430
Susan A, Yadav MK, Kar S, et al. (2019), Molecular identification of blast resistance genes in rice landraces from northeastern India. Plant Pathol 68: 537-546. doi: 10.1111/ppa.12975
Takeda T, Takahashi M, Shimizu M, et al. (2021) Apoplastic CBM1-interacting proteins bind conserved carbohydrate binding module 1 motifs in fungal hydrolases to counter pathogen invasion. bioRxiv 2021.12.31.474618; doi: 10.1101/2021.12.31.474618
Takeda T, Takahashi M, Shimizu M, et al. (2022) Rice apoplastic CBM1-interacting protein counters blast pathogen invasion by binding conserved carbohydrate binding module 1 motif of fungal proteins. PLoS Pathog 18(9): e1010792. doi: 10.1371/journal.ppat.1010792
Tang W, Gao C, Wang J, et al. (2018) Disruption of actin motor function due to MoMyo5 mutation impairs host penetration and pathogenicity in Magnaporthe oryzae. Molecular Plant Pathology 19: 689-699. doi: 10.1111/mpp.12554
TeBeest DO, Guerber C, Ditmore M (2007) Rice blast. The Plant Health Instructor. Reviewed 2012. doi: 10.1094/PHI-I-2007-0313-07
Telebanco‐Yanoria MJ, Ohsawa R, Senoo S, et al. (2008) Diversity analysis for resistance of rice (Oryza sativa L.) to blast disease [Magnaporthe grisea (Hebert) Barr.] using differential isolates from the Philippines. Plant Breeding, 127: 355-363. doi: 10.1111/j.1439-0523.2008.01497.x
Titone P, Mongiano G, Tamborini L (2015) Resistance to neck blast caused by Pyricularia oryzae in Italian rice cultivars. European Journal of Plant Pathology 142(1): 49–59. doi: 10.1007/s10658-014-0588-1
Urashima AS, Alves AF, Silva FN, et al. (2017) Host range, mating type and population structure of Magnaporthe sp. of a single barley field in São Paulo state, Brazil. Journal of Phytopathology 165: 414–424. doi: 10.1111/jph.12575
Valent B (2021) The Impact of Blast Disease: Past, Present, and Future. Methods Mol Biol. 2356: 1-18. doi: 10.1007/978-1-0716-1613-0_1
Velmurugan S, Ashajyothi M, Charishma K, et al. (2023) Enhancing defense against rice blast disease: Unveiling the role of leaf endophytic firmicutes in antifungal antibiosis and induced systemic resistance. Microb Pathog.: 106326. doi: 10.1016/j.micpath.2023.106326
Vu Q, Dossa GS, Mundaca EA, et al. (2022) Combined Effects of Soil Silicon and Host Plant Resistance on Planthoppers, Blast and Bacterial Blight in Tropical Rice. Insects 13(7): 604. doi: 10.3390/insects13070604
Wang X, Jia Y, Wamishe Y, et al. (2017) Dynamic Changes in the Rice Blast Population in the United States Over Six Decades. Molecular Plant-Microbe Interactions 30(10): 803-812. doi: 10.1094/MPMI-04-17-0101-R
Wang Z, Xia Y, Lin S, et al. (2018) Osa‐miR164a targets OsNAC60 and negatively regulates rice immunity against the blast fungus Magnaporthe oryzae. The Plant Journal 95: 584-597. doi: 10.1111/tpj.13972
Wang S, Wang J, Zhang Z, et al. (2021) The risk of wheat blast in rice-wheat-co-planting regions in China: MoO strains of Pyricularia oryzae cause typical symptom and host reaction on both wheat leaves and spikes. Phytopathology. doi: 10.1094/PHYTO-10-20-0470-R
Wang Y, Wang Z, Jibril SM, et al. (2022) Transcriptome and Quasi-Targeted Metabolome Analyze Overexpression of 4-Hydroxyphenylpyruvate Dioxygenase Alleviates Fungal Toxicity of 9-Phenanthrol in Magnaporthe oryzae. Int J Mol Sci. 23(13): 7116. doi: 10.3390/ijms23137116
Wang Q, Wang J, Huang P, et al. (2022) Nucleosome Assembly Protein 1, Nap1, Is Required for the Growth, Development, and Pathogenicity of Magnaporthe oryzae. Int J Mol Sci. 23(14): 7662. doi: 10.3390/ijms23147662
Wang B, Li H, Chen T, et al. (2022) Two new sesquiterpene derivatives, dendocarbin B and bisaborosaol C with antifungal activity from the endophytic fungus Nigrospora chinensis GGY-3. Nat Prod Res.: 1-9. doi: 10.1080/14786419.2022.2151011
Wang L, Zhang X, Li L, et al. (2023) A key sphingolipid pathway gene, MoDES1, regulates conidiation, virulence and plasma membrane tension in Magnaporthe oryzae. Microbiol Res. 279: 127554. doi: 10.1016/j.micres.2023.127554
Wei YY, Liang S, Zhang YR, et al. (2020) MoSec61β, the beta subunit of Sec61, is involved in fungal development and pathogenicity, plant immunity, and ER-phagy in Magnaporthe oryzae. Virulence 11(1): 1685-1700. doi: 10.1080/21505594.2020.1848983
Wei YY, Liang S, Zhu XM, et al. (2023) Recent Advances in Effector Research of Magnaporthe oryzae. Biomolecules 13(11): 1650. doi: 10.3390/biom13111650
Wegner A, Casanova F, Loehrer M, et al. (2022) Gene deletion and constitutive expression of the pectate lyase gene 1 (MoPL1) lead to diminished virulence of Magnaporthe oryzae. J Microbiol. 60(1): 79-88. doi: 10.1007/s12275-022-1074-7
Were V, Yan X, Foster AJ, et al. (2024) The blast effector Pwl2 is a virulence factor that modifies the cellular localisation of host protein HIPP43 to suppress immunity. bioRxiv 2024.01.20.576406; doi: 10.1101/2024.01.20.576406
Wockenfuss A, Chan K, Cooper JG, et al. (2024) A Bacillus velezensis strain shows antimicrobial activity against soilborne and foliar fungi and oomycetes. Front Fungal Biol. 5: 1332755. doi: 10.3389/ffunb.2024.1332755
Wu X, Chen Y, Li C, et al. (2021) GroEL protein from the potential biocontrol agent Rhodopseudomonas palustris enhances resistance to rice blast disease. Pest Manag Sci. doi: 10.1002/ps.6584
Wu MH, Yu Q, Tao TY, et al. (2022) Genome-Wide Analysis of AGC Kinases Reveals that MoFpk1 Is Required for Development, Lipid Metabolism, and Autophagy in Hyperosmotic Stress of the Rice Blast Fungus Magnaporthe oryzae. mBio.:e0227922. doi: 10.1128/mbio.02279-22
Wu XY, Dong B, Zhu XM, et al. (2023) SP-141 targets Trs85 to inhibit rice blast fungus infection and functions as a potential broad-spectrum antifungal. Plant Commun.: 100724. doi: 10.1016/j.xplc.2023.100724
Wu S, Zhang Y, Xu L, et al. (2024) Mitochondrial outer membrane translocase MoTom20 modulates mitochondrial morphology and is important for infectious growth of the rice blast fungus. Mol Plant Microbe Interact. 2024 Jan 3. doi: 10.1094/MPMI-10-23-0168-R
, , , (2022) The activity of the RGA5 sensor NLR from rice requires binding of its integrated HMA domain to effectors but not HMA domain self-interaction. Molecular Plant Pathology. doi: 10.1111/mpp.13236
Xiang Z, Okada D, Asuke S, et al. (2022) Novel insights into host specificity of Pyricularia oryzae and Pyricularia grisea in the infection of gramineous plant roots. Mol Plant Pathol. 23(11): 1658-1670. doi: 10.1111/mpp.13259
, , , et al. (2022) The Piks allele of the NLR immune receptor Pik breaks the recognition of AvrPik effectors of the rice blast fungus. J. Integr. Plant Biol. 00: 0– 0. doi: 10.1111/jipb.13375
Xie P, Liu J, Lu R, et al. (2022) Molecular evolution of the Pi-d2 gene conferring resistance to rice blast in Oryza. Front. Genet. 13:991900. doi: 10.3389/fgene.2022.991900
Xie Y, Wang Y, Yu X, et al. (2022) SH3P2, a SH3 domain-containing protein that interacts with both Pib and AvrPib, suppresses effector-triggered, Pib-mediated immunity in rice. Mol Plant.: S1674-2052(22)00374-4. doi: 10.1016/j.molp.2022.10.022
Yadav R, Ramakrishna W (2023) MicroRNAs Involved in Nutritional Regulation During Plant-Microbe Symbiotic and Pathogenic Interactions with Rice as a Model. Mol Biotechnol. doi: 10.1007/s12033-023-00822-y
Yan X, Ma WB, Li Y, et al. (2011) A sterol 14α-demethylase is required for conidiation, virulence and for mediating sensitivity to sterol demethylation inhibitors by the rice blast fungus Magnaporthe oryzae. Fungal Genetics and Biology 48: 144-153. doi: 10.1016/j.fgb.2010.09.005
Yan X, Tang B, Ryder L, et al. (2022) The transcriptional landscape of plant infection by the rice blast fungus Magnaporthe oryzae reveals distinct families of temporally co-regulated and structurally conserved effectors. bioRxiv 2022.07.18.500532; doi: 10.1101/2022.07.18.500532
Yang C, Liu R, Pang J, et al. (2021) Poaceae-specific cell wall-derived oligosaccharides activate plant immunity via OsCERK1 during Magnaporthe oryzae infection in rice. Nature Communications 12: 2178. doi: 10.1038/s41467-021-24162-0
Yin Z, Liu X, Huang J, Kou Y and Ding X (2023) Editorial: Virulence of filamentous fungi and its interaction with plants. Front. Cell. Infect. Microbiol. 13:1168148. doi: 10.3389/fcimb.2023.1168148
You X, Zhang F, Liu Z, et al. (2022) Rice catalase OsCATC is degraded by E3 ligase APIP6 to negatively regulate immunity. Plant Physiol.: kiac317. doi: 10.1093/plphys/kiac317
Yu M, Zhou Z, Liu X, et al. (2021) The OsSPK1-OsRac1-RAI1 defense signaling pathway is shared by two distantly related NLR proteins in rice blast resistance. Plant Physiol. 28:kiab445. doi: 10.1093/plphys/kiab445
Zdrzałek R, Xi Y, Langner T, et al. (2024) Bioengineering a plant NLR immune receptor with a robust binding interface towards a conserved fungal pathogen effector. bioRxiv 2024.01.20.576400; doi: 10.1101/2024.01.20.576400
Zhang H, Wu Z, Wang C, Li Y, Xu JR (2014) Germination and infectivity of microconidia in the rice blast fungus Magnaporthe oryzae. Nature Communications 5: 4518. doi: 10.1038/ncomms5518
Zhang W, Huang J, Cook DE (2021) Histone modification dynamics at H3K27 are associated with altered transcription of in planta induced genes in Magnaporthe oryzae. PLoS Genet 17(2): e1009376. doi: 10.1371/journal.pgen.1009376
, , , et al. (2021) MoSep3 and MoExo70 are needed for MoCK2 ring assembly essential for appressorium function in the rice blast fungus, Magnaporthe oryzae. Mol Plant Pathol. 00: 1– 6. doi: 10.1111/mpp.13092
Zhang C, He J, Dai H, et al. (2021) Discriminating symbiosis and immunity signals by receptor competition in rice. Proc Natl Acad Sci U S A. 118(16): e2023738118. doi: 10.1073/pnas.2023738118
Zhang Z, Hao Z, Chai R, et al. (2022) Adenylsuccinate Synthetase MoADE12 Plays Important Roles in the Development and Pathogenicity of the Rice Blast Fungus. J Fungi (Basel) 8(8):780. doi: 10.3390/jof8080780
Zhang L, Shan C, Zhang Y, et al. (2022) Transcriptome Analysis of Protein Kinase MoCK2, which Affects Acetyl-CoA Metabolism and Import of CK2-Interacting Mitochondrial Proteins into Mitochondria in the Rice Blast Fungus Magnaporthe oryzae. Microbiol Spectr.: e0304222. doi: 10.1128/spectrum.03042-22
Zhang X, Guo B, Wang Y, et al. (2022) A Detection Method for Crop Fungal Spores Based on Microfluidic Separation Enrichment and AC Impedance Characteristics. J Fungi (Basel) 8(11): 1168. doi: 10.3390/jof8111168
Zhang G, Li R, Wu X, Li M (2023) Natural Product Aloesin Significantly Inhibits Spore Germination and Appressorium Formation in Magnaporthe oryzae. Microorganisms 11(10): 2395. doi: 10.3390/microorganisms11102395
Zhang J, Li H, Gu W, et al. (2023) Peroxisome dynamics determines host-derived ROS accumulation and infectious growth of the rice blast fungus. mBio.: e0238123. doi: 10.1128/mbio.02381-23
Zhang H, Jiao J, Zhao T, et al. (2023) GERWR: Identifying the Key Pathogenicity-associated sRNAs of Magnaporthe oryzae Infection in Rice Based on Graph Embedding and Random Walk with Restart. IEEE/ACM Trans Comput Biol Bioinform. PP. doi: 10.1109/TCBB.2023.3348080
Zheng C, Liu Y, Sun F, et al. (2021) Predicting Protein–Protein Interactions Between Rice and Blast Fungus Using Structure-Based Approaches. Front. Plant Sci. 12: 690124. doi: 10.3389/fpls.2021.690124
Zheng H, , , et al.
Zheng L, Yu Y, Zheng Y, et al. (2023) Long small RNA76113 targets CYCLIC NUCLEOTIDE-GATED ION CHANNEL 5 to repress disease resistance in rice. Plant Physiol.: kiad599. doi: 10.1093/plphys/kiad599
Zhong X, Yang J, Shi Y, et al. (2018) The DnaJ protein OsDjA6 negatively regulates rice innate immunity to the blast fungus Magnaporthe oryzae. Molecular Plant Pathology 19: 607-614. doi: 10.1111/mpp.12546
Zhong Z, Chen M, Lin L, et al. (2018) Population genomic analysis of the rice blast fungus reveals specific events associated with expansion of three main clades. The ISME Journal. doi: 10.1038/s41396-018-0100-6
Zhou E, Jia Y, Singh P, et al. (2007) Instability of the Magnaporthe oryzae avirulence gene AVR-Pita alters virulence. Fungal Genetics and Biology 44(10): 1024-1034. doi: 10.1016/j.fgb.2007.02.003
, , , et al. (2021) The COMPASS‐like complex modulates fungal development and pathogenesis by regulating H3K4me3‐mediated targeted gene expression in Magnaporthe oryzae. Molecular Plant Pathology 22: 422– 439. doi: 10.1111/mpp.13035
Zhou H, Hwarari D, Zhang Y, et al. (2022) Proteomic Analysis Reveals Salicylic Acid as a Pivotal Signal Molecule in Rice Response to Blast Disease Infection. Plants (Basel). 11(13): 1702. doi: 10.3390/plants11131702
Zhou H, Deng J, Cai D, et al. (2022) Effects of Image Dataset Configuration on the Accuracy of Rice Disease Recognition Based on Convolution Neural Network. Front Plant Sci. 13: 910878. doi: 10.3389/fpls.2022.910878
, , Tandem DNA repeats contain cis‐regulatory sequences that activate biotrophy‐specific expression of Magnaporthe effector gene PWL2. Molecular Plant Pathology 00: 1– 14. doi: 10.1111/mpp.13038
Zhu F, Jia Y, Tian C, et al. (2021) Bacillus subtilis GB519 Promotes Rice Growth and Reduces the Damages Caused by Rice Blast Fungus Magnaporthe oryzae. PhytoFrontiers™ 1:4, 330-338. doi: 10.1094/PHYTOFR-12-20-0041-R