.
Condición fitosanitaria: Presente
Grupo de cultivos: Oleaginosas
Especie hospedante: Soja (Glycine max)
Rango de hospedantes: amplio, no específico
Epidemiología: monocíclica, subaguda
Etiología: Hongo. Necrotrófico
Agente causal:
Fusarium virguliforme O’Donnell & T. Aoki,
Fusarium tucumaniae T. Aoki, O’Donnell, Yos. Homma & Lattanzi,
Fusarium brasiliense T. Aoki & O’Donnell,
Fusarium crassistipitatum Scandiani, T. Aoki & O´Donnell, sp. nov. (Aoki et al., 2005)
.
Taxonomía: Eukaryota > Fungi > Dikarya > Ascomycota > Pezizomycotina > Sordariomycetes > Hypocreales > Nectriaceae > Fusarium
.
.
.
Síntomas
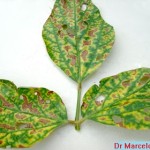
Los primeros síntomas del síndrome de la muerte súbita (SMS) de la soja, conocido en inglés como soybean sudden death syndrome (SDS), generalmente se manifiestan a partir de floración en adelante, aunque pueden observarse en etapas vegetativas, y consisten en clorosis con posterior necrosis internerval (semejantes a las causadas por el cancro y la pudrición por Phialophora gregata).
.
- Síntomas de la muerte súbita de la soja (necrosis y clorosis internerval), causados por Fusarium spp. Autor: Daren Muller
.
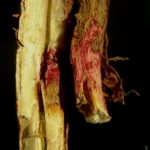
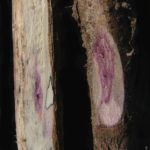
Frecuentemente se produce la caída de los folíolos, quedando los pecíolos adheridos al tallo. Los síntomas foliares progresan rápidamente, de aquí el nombre de muerte súbita. El sistema radicular es afectado y se observa menor desarrollo y podredumbre de raíces, lo cual determina que las plantas afectadas puedan ser fácilmente arrancadas del suelo. En infecciones severas puede observarse manchado rojizo que puede rodear el cuello de la planta. El tejido externo se pudre, pero la médula permanece blanca. Los síntomas pueden ser confundidos con la podredumbre del tallo causada por Phialophora gregata. La clave en la detección de esta enfermedad es que el tejido vascular puede tornarse marrón rojizo, pero la médula permanece siempre blanca. Las plantas se marchitan, mueren prematuramente en forma aislada pero más comúnmente en grupo o a veces distribuidas por todo el campo.
- Síntomas foliares característicos del síndrome de la muerte súbita de la soja. Autor: Daren Mueller.
- El síndrome de la muerte súbita es a menudo la causa de la clorosis intervenal de las hojas de soja, aunque este síntoma puede tener otra causa. Autor: Albert Tenuta.
- Síntomas foliares del síndrome de la muerte súbita de la soja. Autor: B. Kleinke
- Autor: Dirceu Gassen
.
Condiciones ambientales predisponentes para el establecimiento de la enfermedad
Suelos productivos, fértiles, cultivos bajo siembra directa con muchos años de soja sin rotación, húmedos parecen reunir condiciones predisponentes para la enfermedad. La severidad es mayor con tiempo fresco, húmedo y suelos compactados, con drenaje deficiente. A menudo está asociado a nematodes (Heterodera glycines).
.
- Muerte subita de la soja (Fusarium spp.)
- Muerte subita de la soja (Fusarium spp.)
- Autor: Dirceu Gassen
- Autor: Dirceu Gassen
- Autor: Dirceu Gassen
- Autor: Dirceu Gassen
- Autor: Tom Allen
- Autor: Tom Allen
.
Antecedentes y Daños
El síndrome de la muerte súbita o repentina (SMS) es una importante enfermedad fúngica de la soja (Leandro et al., 2012). En la Argentina fue identificada por primera vez en la región pampeana norte, durante la campaña 1991/92, y en el noroeste argentino (NOA) en la campaña siguiente (Scandiani et al., 2012). Progresivamente, se convirtió en una de las patologías de mayor importancia con daños en variedades susceptibles entre 15 y 90%, en el C. y N. del país, respectivamente (Ploper, 1993). Los primeros síntomas generalmente se manifiestan a partir de floración, aunque pueden observarse en etapas vegetativas, y consisten en clorosis con posterior necrosis internerval, pudiendo aparecer en estados vegetativos tempranos como V4 (Rupe & Hartman, 1999). Frecuentemente, se produce la caída de los folíolos, quedando los pecíolos adheridos al tallo. El sistema radicular es afectado, exhibiendo pigmentación rojiza en algunas ocasiones, en forma externa y basal, observándose menor desarrollo y podredumbre de raíces. Las plantas se marchitan, mueren en forma aislada, pero más comúnmente en grupos, o a veces distribuidas por todo el campo.
.
- Autor: Jaime Cummings, NYS IPM Program
.
El agente causal del SMS fue conocido primariamente como Fusarium solani f. sp. glycines, posteriormente a estudios filogenéticos moleculares, morfológicos y patogénicos realizados desde 2003, se demostró que es causado por cuatro especies de Fusarium habitantes del suelo muy relacionadas entre sí: F. virguliforme O’Donnell & T. Aoki, F. tucumaniae T. Aoki, O’Donnell, Yos. Homma & Lattanzi, F. brasiliense T. Aoki & O’Donnell, y una especie nueva Fusarium crassistipitatum Scandiani, T. Aoki & O´Donnell, sp. nov. (Aoki et al., 2005). En Argentina se comprobó la existencia de los todos los agentes causales, con prevalencia de F. tucumaniae, seguido por F. virguliforme, siendo esta última la única especie presente en Estados Unidos (Scandiani et al., 2004; O´Donnell et al., 2010; Aoki et al., 2005). En Brasil, estudios efectuados con pocos aislamientos, demostraron que al menos se encuentran presentes F. tucumaniae, F. brasiliense y F. crassistipitatum. En 2006 se demostró la ocurrencia de la forma sexual (peritecios) de F. tucumaniae mediante cruzamientos en condiciones de laboratorio (Covert et al., 2007) y en 2010 se la encontró en la naturaleza en Fontezuela, Buenos Aires (Scandiani et al., 2011).
Las pérdidas en el rendimiento son variables y dependientes del genotipo, ambiente, condiciones edáficas, fecha de siembra, disponibilidad de K, macroporosidad y pH del suelo, biotipos presentes del/los patógenos y posiblemente de otros factores también involucrados (Estevez de Jensen et al., 2001; Leandro et al., 2012); en Argentina se estimaron daños promedio de 1514 kg/ha (entre 192-3770 kg/ha) según Scandiani et al. (2012).
.
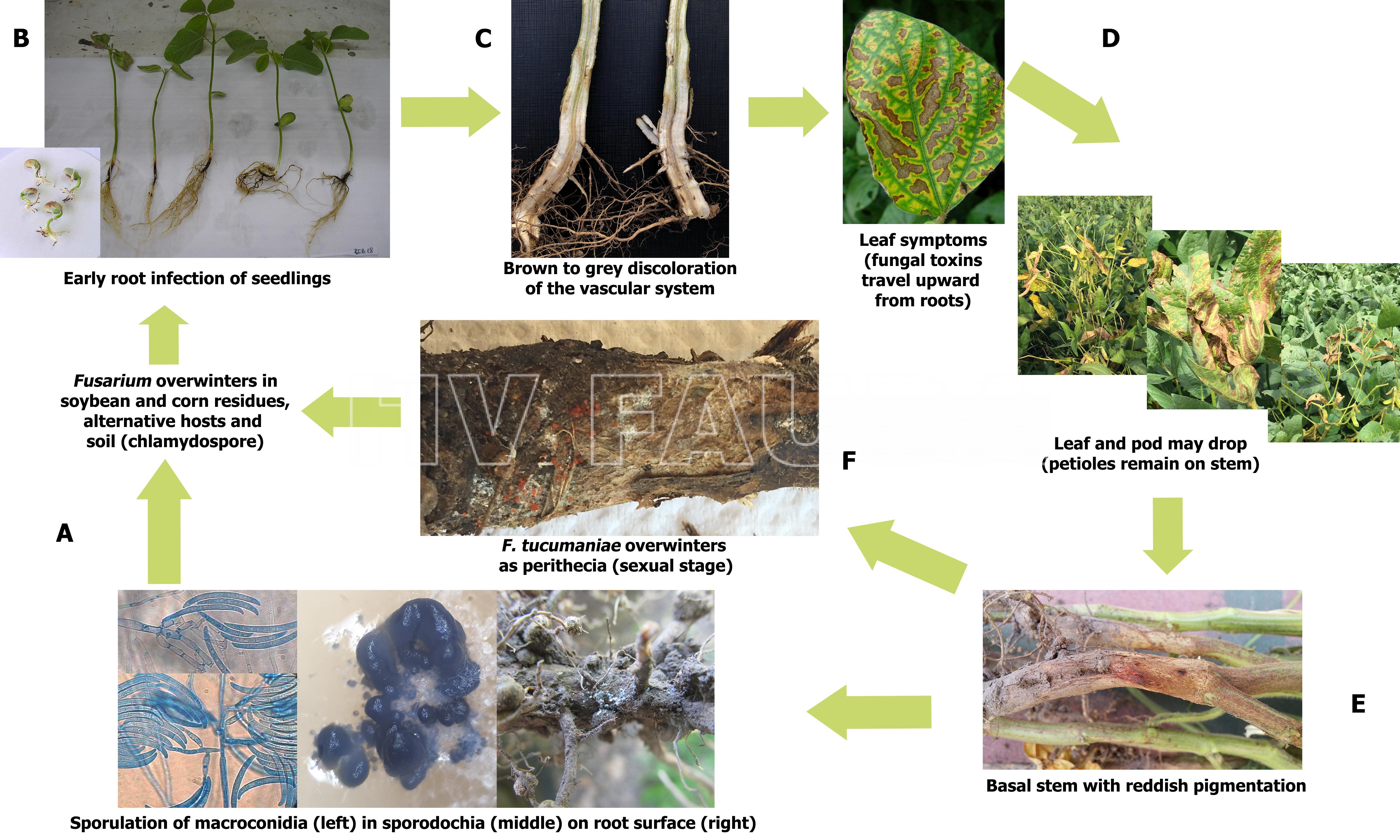
Ciclo de la enfermedad
Las especies de Fusarium tienen varias estrategias de supervivencia, como conidios o clamidosporas en reposo en el suelo, infestación de restos de cultivo (rastrojos) e infección de un amplio rango de hospedantes alternativos. Las clamidosporas de Fusarium pueden sobrevivir en el suelo durante muchos años. La infección es vía radicular.
.
- Ciclo biológico agronómico de la muerte súbita de la soja, causado por Fusarium spp. Autor: Rodriguez et al., 2021.
.
Manejo de la enfermedad
La mejor estrategia es la utilización de variedades que tengan mejor comportamiento, y la posibilidad de sembrar en suelos supresivos (aquellos suelos que son capaces naturalmente de inhibir la colonización e infección de patógenos) (Westphal y Xing, 2011). Los suelos compactados también aumentan la intensidad de las enfermedades causadas por Fusarium spp. Por lo tanto, minimizar la compactación del suelo mejora el drenaje y el crecimiento de las raíces de las plantas. Si los granos infectados con Fusarium spp. se utilizan como semilla, los tratamientos de semillas con fungicidas pueden usarse para reducir la podredumbre de las semillas y las enfermedades de las plántulas causadas por Fusarium spp. Sin embargo, el tratamiento de las semillas no es adecuado para el control de la SMS de la soja (Zaworski, 2014).
Otra práctica cultural es la rotación de cultivos incluyendo cultivos no hospedantes, como el trigo con rotación soja-alfalfa, que potencialmente podría reducir el SMS. Sin embargo, la combinación maíz-soja en rotación anual no reduce ni la incidencia ni la severidad de la enfermedad. Rotaciones anuales de maíz y soja (en comparación con otros diseños de rotación plurianuales que incluyen otras gramíneas y forrajeras) no son efectivas para reducir el riesgo del SMS. Xing y Westphal (2009) encontraron que después de rotaciones con maíz, las raíces de soja no estaban visualmente más sanas que las que provenían del monocultivo de soja. Cuando se siembra maíz, la población de patógenos edáficos puede declinar hasta cierto punto, pero no lo suficiente como para reducir la presión de enfermedad cuando se siembre soja al año siguiente. Aunque una rotación de dos años puede mantener las densidades poblacionales del nematodo del quiste de la soja (NQS, causado por Heterodera glycines) por debajo de los umbrales de daño económico, si la densidad poblacional inicial es baja, dicha rotación es demasiado corta como para reducir el riesgo del SMS (Xing y Westphal, 2006; Diaz Arias, 2012; Westphal et al., 2014). Por otro lado, recientemente se ha sugerido que una cosecha de maíz limpia podría reducir el riesgo del SMS, al reducir la colonización de granos de maíz que favorecen la supervivencia de F. virguliforme, mientras que pérdidas considerable de maíz durante la cosecha podrían aumentar el riesgo del SMS (Navi y Yang, 2016). Por lo tanto, el cultivo continuo debe ser evitado si las enfermedades de Fusarium spp. son severas en un campo, especialmente en sistemas bajo siembra directa. De esta manera, en general el SMS de la soja no puede ser controlado por la rotación de cultivos (Marburger et al., 2014).
.
.
.
Videos
Detecting soybean sudden death syndrome using remote sensing Iowa State University
Sudden Death Syndrome of Soybean. Univ of Wisconsin Integrated Pest and Crop Management, Dr. Damon Smith
Sudden Death Syndrome in Soybean. University of Nebraska-Lincoln Plant Pathologist, Loren Giesler
Soybean Disease: Sudden Death Syndrome. Purdue Extension Entm, Dr. Kiersten Wise
Sudden Death Syndrome (SDS) of Soybeans
.
Bibliografía
Sudden Death Syndrome. University of Minnesota Extension
Aoki T, O’Donnell K, Homma Y, Lattanzi AR (2003) Sudden-death syndrome of soybean is caused by two morphologically and phylogenetically distinct species within the Fusarium solani species complex–F. virguliforme in North America and F. tucumaniae in South America. Mycologia 95:660-684.
Aoki T, O’Donnell K, Scandiani MM (2005) Sudden death syndrome of soybean in South America is caused by four species of Fusarium: Fusarium brasiliensesp. nov., F. cuneirostrum sp. nov., F. tucumaniae, and F. virguliforme. Mycoscience 46:162-183. doi: 10.1007/S10267-005-0235-Y
Aoki T, Tanaka F, Suga H, et al. (2012) Fusarium azukicola sp. nov., an exotic azuki bean root-rot pathogen in Hokkaido, Japan. Mycologia 104:1068-1084. doi: 10.3852/11-303
Baetsen-Young AM, Swinton SM, Chilvers MI (2020) Economic Impact of Fluopyram-Amended Seed Treatments to Reduce Soybean Yield Loss Associated with Sudden Death Syndrome. Plant Disease, doi: 10.1094/PDIS-04-20-0792-RE
Baetsen-Young A, Man Wai C, VanBuren R, Day B (2020) Fusarium virguliforme Transcriptional Plasticity Is Revealed by Host Colonization of Maize versus Soybean. Plant Cell. 32(2): 336-351. doi: 10.1105/tpc.19.00697
Bailey RC, Norris RH, Reynoldson TB (2004) Bioassessment of Freshwater Ecosystems: Using the Reference Condition Approach. Springer, New York. doi: 10.1007/978-1-4419-8885-0
Baker KF, Cook RJ (1982) Biological Control of Plant Pathogens. American Phytopathological Society, St. Paul, MN.
Bakker MG, Brown DW, Kelly AC, et al. (2018) Fusarium mycotoxins: a trans-disciplinary overview. Canadian Journal of Plant Pathology 40(2): 161-171. doi: 10.1080/07060661.2018.1433720
Bakkeren G, Kronstad JW, Lévesque CA (2000) Comparison of AFLP fingerprints and ITS sequences as phylogenetic markers in Ustilaginomycetes. Mycologia 92:510-521.
Brar HK, Swaminathan S, Bhattacharyya MK (2011) The Fusarium virguliforme toxin FvTox1 causes foliar sudden death syndrome-like symptoms in soybean. Mol Plant Microbe Interact. 24(10): 1179-88. doi: 10.1094/MPMI-12-10-0285
Brar HK, Bhattacharyya MK (2012) Expression of a single-chain variable-fragment antibody against a Fusarium virguliforme toxin peptide enhances tolerance to sudden death syndrome in transgenic soybean plants. Mol Plant Microbe Interact. 25(6):817-24. doi: 10.1094/MPMI-12-11-0317
Benitez T, Rincon AM, Limon MC, Codon AC (2004) Biocontrol mechanisms of Trichoderma strains. Int. Microbiol. 7:249-260.
Bergmann GT, Walters WA, Knight R, et al. (2011) The under-recognized dominance of Verrucomicrobia in soil bacterial communities. Soil Biol. Biochem. 43:1450-1455. doi: 10.1016/j.soilbio.2011.03.012
Berka RM, Grigoriev IV, Otillar R, et al. (2011) Comparative genomic analysis of the thermophilic biomass-degrading fungi Myceliophthora thermophila and Thielavia terrestris. Nat. Biotechnol. 29:922-927. doi: 10.1038/nbt.1976
Bissonnette KM, Barizon J, Adee EA, et al. (2024) Management of soybean cyst nematode and sudden death syndrome with nematode-protectant seed treatments across multiple environments in soybean. Plant Disease. doi: 10.1094/PDIS-02-23-0292-RE
Blaxter M (1998) Caenorhabditis elegans is a nematode. Science 282:2041. doi: 10.1126/science.282.5396.2041
Bowers JH, Kinkel LL, Jones RK (1996) Influence of disease-suppressive strains of Streptomyces on the native Streptomyces community in soil as determined by the analysis of cellular fatty acids. Can. J. Microbiol. 42:27-37. doi: 10.1139/m96-005
Brar HK, Swaminathan S, Bhattacharyya MK (2011) The Fusarium virguliforme toxin FvTox1 causes foliar sudden death syndrome-like symptoms in soybean. Mol Plant Microbe. 24: 1179–1188. doi: 10.1094/MPMI-12-10-0285
Broadbent P, Baker KF (1974) Behavior of Phytophthora cinnamomi in soils suppressive and conducive to root rot. Aust. J. Agric. Res. 25:121. doi: 10.1071/AR9740121
Brown MT, Oh S, Rainey KM, Telenko DEP (2024) Presymptomatic Leaf Reflectance of Fusarium virguliforme-Infected Soybean Plants in Greenhouse Conditions. PhytoFrontiers. doi: 10.1094/PHYTOFR-09-23-0121-R
Carmona M, Alvarez D, Luque A, et al. (2011) Daños asociados al síndrome de la muerte súbita en el cultivo de soja. 5° Congreso de la soja del Mercosur. 1° Foro de la soja Asia. 14 al 15 de septiembre de 2011, Rosario, Santa Fe, Argentina.
Castillo Lopez D, Zhu-Salzman K, Ek-Ramos MJ, Sword GA (2014) The entomopathogenic fungal endophytes Purpureocillium lilacinum (formerly Paecilomyces lilacinus) and Beauveria bassiana negatively affect cotton aphid reproduction under both greenhouse and field conditions. PLoS One 9:e103891. doi: 10.1371/journal.pone.0103891
Caswell EP, McKinney HE, Thomason IJ (1985) Extraction of cysts and eggs of Heterodera schachtii from soil with an assessment of extraction efficiency. J. Nematol. 17:337-340.
Cha JY, Han S, Hong HJ, et al. (2016) Microbial and biochemical basis of a Fusarium wilt-suppressive soil. ISME J. 10:119-129. doi: 10.1038/ismej.2015.95
Chang HX, Domier LL, Radwan O, et al. (2016) Identification of Multiple Phytotoxins Produced by Fusarium virguliforme Including a Phytotoxic Effector (FvNIS1) Associated With Sudden Death Syndrome Foliar Symptoms. Mol Plant Microbe Interact. 29(2): 96-108. doi: 10.1094/MPMI-09-15-0219-R
Chang HX, Yendrek CR, Caetano-Anolles G, et al. (2016) Genomic characterization of plant cell wall degrading enzymes and in silico analysis of xylanses and polygalacturonases of Fusarium virguliforme . BMC Microbiology 16: 147. doi: 10.1186/s12866-016-0761-0
Chang HX, Roth MG, Wang D, et al. (2018) Integration of sudden death syndrome resistance loci in the soybean genome. Theoretical and Applied Genetics 1–17. doi: 10.1007/s00122-018-3063-0
Chang HX, Tan R, Hartman GL, et al. (2019) Characterization of Soybean STAY-GREEN Genes in Susceptibility to Foliar Chlorosis of Sudden Death Syndrome. Plant Physiol. 180(2): 711-717. doi: 10.1104/pp.19.00046
Chang HX, Wen Z, Tan R, et al. (2020) Linkage Mapping for Foliar Necrosis of Soybean Sudden Death Syndrome. Phytopathology 110(4): 907-915. doi: 10.1094/PHYTO-09-19-0330-R
Chen SY (2007) Suppression of Heterodera glycines in soils from fields with long-term soybean monoculture. Biocontrol Sci. Technol. 17:125-134. doi: 10.1080/09583150600937121
Chet I, Baker R (1980) Induction of suppressiveness to Rhizoctonia solani in soil. Phytopathology 70:994-998. doi: 10.1094/Phyto-70-994
Chilvers M (2019) Risk prediction tools and the interaction of soybean sudden death syndrome and soybean cyst nematode. Crops & Soils 52: 18-19. doi: 10.2134/cs2019.52.0402
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 18:117-143. doi: 10.1111/j.1442-9993.1993.tb00438.x
Clarke KR, Somerfield PJ, Gorley RN (2008) Testing of null hypotheses in exploratory community analyses: Similarity profiles and biota-environment linkage. J. Exp. Mar. Biol. Ecol. 366:56-69. doi: 10.1016/j.jembe.2008.07.009
Clarke KR, Warwick RM (2001) Change in marine communities: An approach to statistical analysis and interpretation. PRIMER-E Ltd., Plymouth.
Cook RJ, Rovira AD (1976) The role of bacteria in the biological control of Gaeumannomyces graminis by suppressive soils. Soil Biol. Biochem. 8:269-273.
Costa SS, Matos KS, Tessmann DJ, et al. (2016) Fusarium paranaense sp. nov., a member of the Fusarium solani species complex causes root rot on soybean in Brazil. Fungal Biology 120: 51-60. doi: 10.1016/j.funbio.2015.09.005
Covert S, Aoki T, O´Donnell K, et al. (2007) Sexual reproduction in the Sudden Death Syndrome pathogen Fusarium tucumaniae. Fungal Genetics and Biology 44:799-807.
Crous et al. (2022) Fusarium and allied fusarioid taxa (FUSA). Fungal Systematics and Evolution. doi: 10.3114/fuse.2022.09.08
Curl E (1963) Control of plant diseases by crop rotation. Bot. Rev. 29:413. doi: 10.1007/BF02860813
Diaz Arias, Maria Mercedes (2012) Fusarium species infecting soybean roots: Frequency, aggressiveness, yield impact and interaction with the soybean cyst nematode. Graduate Theses and Dissertations. 12314.
de Farias Neto AL, Hashmi R, Schmidt M, et al. (2007) Mapping and confirmation of a new sudden death syndrome resistance QTL on linkage group D2 from the soybean genotypes PI 567374 and ‘Ripley.’ Mol Breed. 20: 53–62. doi: 10.1007/s11032-006-9072-8
De La Torre JR, Walker CB, Ingalls AE, et al. (2008) Cultivation of a thermophilic ammonia oxidizing archaeon synthesizing crenarchaeol. Environ. Microbiol. 10:810-818. doi: 10.1111/j.1462-2920.2007.01506.x
Desjardins AE, Plattner RD (2000) Fumonisin B1-nonproducing strains of Fusarium verticillioides cause maize (Zea mays) ear infection and ear rot. Journal of Agricultural and Food Chemistry 48: 5773–5780. doi: 10.1021/jf000619k
Faghihi J, Ferris JM (2000) An efficient new device to release eggs from Heterodera glycines. J. Nematol. 32:411-413.
Fravel D, Olivain C, Alabouvette C (2003) Fusarium oxysporum and its biocontrol. New Phytol. 157:493-502. doi: 10.1046/j.1469-8137.2003.00700.x
Gao X, Jackson TA, Hartman GL, Niblack TL (2006) Interactions between the soybean cyst nematode and Fusarium solani f. sp. glycines based on greenhouse factorial experiments. Phytopathology 96:1409-1415. doi: 10.1094/PHYTO-96-1409
Geiser DM, Jimenez-Gasco MD, Kang SC, et al. (2004) FUSARIUM-ID v. 1.0: A DNA sequence database for identifying Fusarium. Eur. J. Plant Pathol. 110:473-479. doi: 10.1023/B:EJPP.0000032386.75915.a0
Geiser DM, et al. (2020) Phylogenomic analysis of a 55.1 kb 19-gene dataset resolves a monophyletic Fusarium that includes the Fusarium solani Species Complex. Phytopathology. doi: 10.1094/PHYTO-08-20-0330-LE
Giachero ML, Marquez N, Gallou A, et al. (2017) An In Vitro Method for Studying the Three-Way Interaction between Soybean, Rhizophagus irregularis and the Soil-Borne Pathogen Fusarium virguliforme. Front. Plant Sci. 8:1033. doi: 10.3389/fpls.2017.01033
Gongora-Canul CC, Leandro LFS (2011) Effect of soil temperature and plant age at time of inoculation on progress of root rot and foliar symptoms of soybean sudden death syndrome. Plant Disease 95: 436-440. doi: 10.1094/PDIS-07-10-0489
Gongora-Canul C, Nutter FW, Leandro LFS (2012) Temporal dynamics of root and foliar severity of soybean sudden death syndrome at different inoculum densities. European Journal of Plant Pathology 132(1): 71-79. doi: 10.1007/s10658-011-9849-4
Gray LE, Achenbach LA (1996) Severity of foliar symptoms and root and crown rot of soybean inoculated with various isolates and inoculum rates of Fusarium solani. Plant Dis. 80:1197-1199. doi: 10.1094/PD-80-1197
Haas D, Défago G (2005) Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat. Rev. Microbiol. 3:307-319. doi: 10.1038/nrmicro1129
Hamilton R, Jacobs JL, McCoy AG, et al. (2023) Multistate sensitivity monitoring of Fusarium virguliforme to the SDHI fungicides fluopyram and pydiflumetofen in the United States. Plant Dis. doi: 10.1094/PDIS-11-23-2465-RE
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4:9.
Han JS, Cheng JH, Yoon TM, et al. (2005) Biological control agent of common scab disease by antagonistic strain Bacillus sp. sunhua. J. Appl. Microbiol. 99:213-221. doi: 10.1111/j.1365-2672.2005.02614.x
Hartman GL, Bowen CR, Haudenshield JS, et al. (2015).Evaluation of disease and pest damage on soybean cultivars released from 1923 through 2008 under field conditions in central Illinois. Agron. J. 107:2373-2380. doi: 10.2134/agronj15.0075
Hartman GL, Chang HX, Leandro LF (2015) Research advances and management of soybean sudden death syndrome. Crop Prot. 73:60-66. doi: 10.1016/j.cropro.2015.01.017
Hashimoto M, Murakami T, Funahashi K, et al. (2006) An RNA polymerase inhibitor, cyclothiazomycin B1, and its isomer. Bioorg. Med. Chem. 14:8259-8270. doi: 10.1016/j.bmc.2006.09.006
Herrmann I, Vosberg SK, Ravindran P, et al. (2018) Leaf and Canopy Level Detection of Fusarium Virguliforme (Sudden Death Syndrome) in Soybean. Remote Sensing 10(3): 426. doi: 10.3390/rs10030426
Hirrel MC (1983) Sudden death syndrome of soybean—A disease of unknown etiology. Phytopathology 73: 501-502.
Hoper H, Alabouvette C (1996) Importance of physical and chemical soil properties in the suppressiveness of soils to plant diseases. Eur. J. Soil Biol. 32:41-58.
Howard DD, Chambers AY, Brawley PW, Bush TD (1992) Fertilization effects on soybean sudden death syndrome. Better Crops Plant Food 77:14-15.
Hughes TJ, O’Donnell K, Sink S, et al. (2014) Genetic architecture and evolution of the mating type locus in fusaria that cause soybean sudden death syndrome and bean root rot. Mycologia 106:686-697. doi: 10.3852/13-318
Huson DH, Auch AF, Qi J, Schuster SC (2007) MEGAN analysis of metagenomic data. Genome Res. 17:377-386. doi: 10.1101/gr.5969107
Huson DH, Weber N (2013) Microbial community analysis using MEGAN. Methods Enzymol. 531:465-485. doi: 10.1016/B978-0-12-407863-5.00021-6
Islam KT, Bond JP, Riggs J, Fakhoury AM (2015) Impact of ILeVO on the infection and colonization of soybean roots by Fusarium virguliforme. The American Phytopathological Society Annual Meeting, Pasadena, CA.
Islam KT, Bond JP, Fakhoury AM (2017) FvSNF1, the sucrose non-fermenting protein kinase gene of Fusarium virguliforme, is required for cell-wall-degrading enzymes expression and sudden death syndrome development in soybean. Curr Genet. 63(4): 723-738. doi: 10.1007/s00294-017-0676-9
Islam KT, Bond JP, Fakhoury AM (2017) FvSTR1, a striatin orthologue in Fusarium virguliforme, is required for asexual development and virulence. Appl Microbiol Biotechnol. 101(16): 6431-6445. doi: 10.1007/s00253-017-8387-1
James TY, Letcher PM, Longcore JE, et al. (2006) A molecular phylogeny of the flagellated fungi (Chytridiomycota) and description of a new phylum (Blastocladiomycota). Mycologia 98:860-871. doi: 10.1080/15572536.2006.11832616
Ji J, Scott MP, Bhattacharyya MK (2006) Light is essential for degradation of ribulose-1,5-bisphosphate carboxylase-oxygenase large subunit during sudden death syndrome development in soybean. Plant Biol (Stuttg). 8(5): 597-605. doi: 10.1055/s-2006-924175
Kandel YR, Haudenshield JS, Srour AY, et al. (2015) Multilaboratory comparison of quantitative PCR assays for detection and quantification of Fusarium virguliforme from soybean roots and soil. Phytopathology 105:1601-1611. doi: 10.1094/PHYTO-04-15-0096-R
Kandel YR, Bradley CA, Wise KA, et al. (2015) Effect of Glyphosate Application on Sudden Death Syndrome of Glyphosate-Resistant Soybean Under Field Conditions. Plant Dis. 99(3):347-354. doi: 10.1094/PDIS-06-14-0577-RE
Kandel YR, Wise KA, Bradley CA, et al. (2016) Effect of Planting Date, Seed Treatment, and Cultivar on Plant Population, Sudden Death Syndrome, and Yield of Soybean. Plant Disease 100(8): 1735-1743. doi: 10.1094/PDIS-02-16-0146-RE
Kandel YR, Bradley CA, Chilvers MI, et al. (2020) Relationship Between Sudden Death Syndrome caused by Fusarium virguliforme and Soybean Yield: A Meta-Analysis. Plant Dis. 104(6): 1736-1743. doi: 10.1094/PDIS-11-19-2441-RE
Kannan R, Veeravel R (2012) Effect of different dose and application methods of Paecilomyces lilacinus (Thom.) Samson against root knot nematode, Meloidogyne incognita (Kofoidand White) Chitwood in okra. J. Agric. Sci. 4:119.
Kantartzi SK, Klein J, Schmidt M (2012) Registration of ‘Saluki 4411’ soybean with resistance to sudden death syndrome and HG type 0 (race 3) soybean cyst nematode. J. Plant Registrations 6:298-301. doi: 10.3198/jpr2011.10.0569crc
Kloepper JW, Rodriguez-Kabana R, Zehnder GW, et al. (1999) Plant root-bacterial interactions in biological control of soilborne diseases and potential extension to systemic and foliar diseases. Australas. Plant Pathol. 28:21-26. doi: 10.1071/AP99003
Kok CJ, Papert A, Hok-A-Hin CH (2001) Microflora of Meloidogyne egg masses: Species composition, population density and effect on the biocontrol agent Verticillium chlamydosporium (Goddard). Nematology 3:729-734. doi: 10.1163/156854101753625236
Kolander TM, Bienapfl JE, Kurle JE, Malvick DK (2012) Symptomatic and asymptomatic host range of Fusarium virguliforme, the causal agent of soybean sudden death syndrome. Plant Disease 96: 1148-1153. doi: 10.1094/PDIS-08-11-0685-RE
Konneke M, Bernhard AE, de la Torre JR, et al. (2005) Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature 437:543-546. doi: 10.1038/nature03911
Krishnan SV, Anaswara PA, Nampoothiri KM, et al. (2025) Biocontrol Activity of New Lactic Acid Bacteria Isolates Against Fusaria and Fusarium Mycotoxins. Toxins 17(2): 68. doi: 10.3390/toxins17020068
Kwak YS, Weller DM (2013) Take-all of wheat and natural disease suppression: A review. Plant Pathol. J. 29:125-135. doi: 10.5423/PPJ.SI.07.2012.0112
Lario LD, Gonzalez C, Meini MR, et al. (2023) Exploring Soybean Cultivar Susceptibility to Sudden Death Syndrome: Insights into Isoflavone Responses and Biocontrol Potential. Plant Science: 111951. doi: 10.1016/j.plantsci.2023.111951
Leandro LF, Tatalovic N, Luckew A (2012) Soybean sudden death syndrome−Advances in knowledge and disease management. CAB Rev. 7:1-14. doi: 10.1079/PAVSNNR20127053
Lévesque CA, De Cock AWAM (2004) Molecular phylogeny and taxonomy of the genus Pythium. Mycol. Res. 108:1363-1383. doi: 10.1017/S0953756204001431
Li S, Hartman GL (2003) Molecular detection of Fusarium solani f. sp. glycines in soybean roots and soil. Plant Pathology, 52: 74-83. doi: 10.1046/j.1365-3059.2003.00797.x
Li S, Hartman GL, Chen Y (2009) Evaluation of aggressiveness of Fusarium virguliforme isolates that cause soybean sudden death syndrome. J. Plant Pathol. 91:77-86.
Ma C, Borgatta J, Hudson BG, et al. (2020) Advanced material modulation of nutritional and phytohormone status alleviates damage from soybean sudden death syndrome. Nat. Nanotechnol. doi: 10.1038/s41565-020-00776-1
Malvick DK, Bussey KE (2008) Comparative analysis and characterization of the soybean sudden death syndrome pathogen Fusarium virguliforme in the northern United States. Can. J. Plant Pathol.-Rev. Can. Phytopathol. 30:467-476. doi: 10.1080/07060660809507544
Marburger DA, Venkateshwaran M, Conleya SP, et al. (2014) Crop Rotation and Management Effect on Fusarium spp. Populations. Crop Science 55(1): 365-376. doi: 10.2135/cropsci2014.03.0199
Marchant R (1999) How important are rare species in aquatic community ecology and bioassessment? A comment on the conclusions of Cao et al. Limnol. Oceanogr. 44:1840-1842.
Marquez N, Giachero ML, Gallou A, et al. (2019) Transcriptome analysis of mycorrhizal and nonmycorrhizal soybean plantlets upon infection with Fusarium virguliforme, one causal agent of sudden death syndrome. Plant Pathology 68: 470-480. doi: 10.1111/ppa.12964
Mazzola M (2002) Mechanisms of natural soil suppressiveness to soilborne diseases. Antonie van Leeuwenhoek 81:557-564. doi: 10.1023/A:1020557523557
Mazzola M (2004) Assessment and management of soil microbial community structure for disease suppression. Annu. Rev. Phytopathol. 42:35-59. doi: 10.1146/annurev.phyto.42.040803.140408
McLean KS, Lawrence GW (1995) Development of Heterodera glycines as affected by Fusarium solani, the causal agent of sudden death syndrome of soybean. J. Nematol. 27:70-77.
Mendes R, Kruijt M, de Bruijn I, et al. (2011) Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 332:1097-1100. doi: 10.1126/science.1203980
Mitra S, Stark M, Huson DH (2011) Analysis of 16S rRNA environmental sequences using MEGAN. BMC Genomics 12(Suppl. 3S17. doi: 10.1186/1471-2164-12-S3-S17
Mizuhara N, Kuroda M, Ogita A, et al. (2011) Antifungal thiopeptide cyclothiazomycin B1 exhibits growth inhibition accompanying morphological changes via binding to fungal cell wall chitin. Bioorg. Med. Chem. 19:5300-5310. doi: 10.1016/j.bmc.2011.08.010
Modrzewska M, Bryła M, Kanabus J, Pierzgalski A (2022) Trichoderma as a biostimulator and biocontrol agent against Fusarium in the production of cereal crops: opportunities and possibilities. Plant Pathol. doi: 10.1111/ppa.13578
Murakami H, Tsushima S, Shishido Y (2000) Soil suppressiveness to clubroot disease of Chinese cabbage caused by Plasmodiophora brassicae. Soil Biol. Biochem. 32:1637-1642. doi: 10.1016/S0038-0717(00)00079-1
Muyzer G, de Waal EC, Uitterlinden AG (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 59:695-700.
Narula K, Elagamey E, Abdellatef MAE, et al. (2020) Chitosan‐triggered immunity to Fusarium in chickpea is associated with changes in the plant extracellular matrix architecture, stomatal closure and remodeling of the plant metabolome and proteome. Plant J, 103: 561-583. doi: 10.1111/tpj.14750
Navi SS, Yang XB (2016) Impact of Crop Residue and Corn-soybean Rotation on the Survival of Fusarium virguliforme a Causal Agent of Sudden Death Syndrome of Soybean. J Plant Pathol Microbiol 7:330. DOI:10.4172/2157-7471.1000330
Ngaki MN, Wang B, Sahu BB, et al. (2016) Tanscriptomic Study of the Soybean-Fusarium virguliforme Interaction Revealed a Novel Ankyrin-Repeat Containing Defense Gene, Expression of Whose during Infection Led to Enhanced Resistance to the Fungal Pathogen in Transgenic Soybean Plants. PLoS ONE 11(10): e0163106. doi: 10.1371/journal.pone.0163106
Njiti VN, Doubler TW, Suttner RJ, et al. (1998) Resistance to soybean sudden death syndrome and root colonization by Fusarium solani f. sp. Glycine in near-isogenic lines. Crop Sci. 38:472-477. doi: 10.2135/cropsci1998.0011183X003800020033x
Nunn GB, Theisen BF, Christensen B, Arctander P (1996) Simplicity-correlated size growth of the nuclear 28S ribosomal RNA D3 expansion segment in the crustacean order Isopoda. J. Mol. Evol. 42:211-223. doi: 10.1007/BF02198847
O’Donnell K, Sink S, Scandiani M, et al. (2010) Soybean sudden death syndrome species diversity within North and South America revealed by multilocus genotyping. Phytopathology 100: 58-71. doi: 10.1094/PHYTO-100-1-0058
O’Donnell K, Whitaker BK, Laraba I, et al. (2022) DNA Sequence-Based Identification of Fusarium: A Work in Progress. Plant Disease 106(6): 1597-1609. doi: 10.1094/PDIS-09-21-2035-SR
Pawlowski ML, Hartman GL (2020) Reduction of Sudden death syndrome foliar symptoms and Fusarium virguliforme DNA in roots inoculated with rhizophagus intraradices. Plant Dis. 104: 1415–1420. doi: 10.1094/PDIS-07-19-1500-RE
Penton CR, Gupta VVSR, Tiedje JM, et al. (2014) Fungal community structure in disease suppressive soils assessed by 28S LSU gene sequencing. PLoS One 9:e93893. doi: 10.1371/journal.pone.0093893
Perez CDP, La Torre Roche R De, Zuverza-Mena N, et al. (2020) Metalloid and Metal Oxide Nanoparticles Suppress Sudden Death Syndrome of Soybean. J. Agric. Food Chem., 68, 77–87. doi: 10.1021/acs.jafc.9b06082
, , Host‐induced gene silencing for engineering resistance to Fusarium in soybean. Plant Pathology 00: 1– 9. doi: 10.1111/ppa.13299
Pimentel MF, Arnão E, Warner AJ, et al. (2020) Trichoderma Isolates Inhibit Fusarium virguliforme Growth, Reduce Root Rot, and Induce Defense-Related Genes on Soybean Seedlings. Plant Disease 104(7):1949-1959. doi: 10.1094/PDIS-08-19-1676-RE
Pin Viso N, Sautua F, Scandiani M, et al. (2016) In vitro antagonistic activity of native bacteria isolated from soils of the argentine Pampas against Fusarium tucumaniae and Fusarium virguliforme. African Journal of Microbiology Research 10(27): 1031-1035. DOI: 10.5897/AJMR2016.7915
Ploper LD (1993) Síndrome de la muerte súbita: nueva enfermedad de la soja en el noroeste argentino. Avance Agroindustrial 13:5-9.
Pudake RN, Swaminathan S, Sahu BB, et al. (2013) Investigation of the Fusarium virguliforme fvtox1 mutants revealed that the FvTox1 toxin is involved in foliar sudden death syndrome development in soybean. Curr Genet 59: 107–117. doi: 10.1007/s00294-013-0392-z
Pyrowolakis A, Westphal A, Sikora RA, Ole Becker J (2002) Identification of root-knot nematode suppressive soils. Appl. Soil Ecol. 19:51-56. doi: 10.1016/S0929-1393(01)00170-6
Radwan O, Li M, Calla B, et al. (2013) Soybean response to Fv toxin. Molecular Plant Pathology 14: 293-307. doi: 10.1111/mpp.12006
Rairdin A, Fotouhi F, Zhang J, et al. (2022) NAPPN Annual Conference Abstract: Genome wide association study of sudden death syndrome in soybean using deep learning-based phenotyping. Authorea. doi: 10.22541/au.166497104.42473667/v1
Rairdin A, Fotouhi F, Zhang J, et al. (2022) Deep learning-based phenotyping for genome wide association studies of sudden death syndrome in soybean. Front. Plant Sci. 13: 966244. doi: 10.3389/fpls.2022.966244
Raza MM, Harding C, Liebman M, Leandro LF (2020) Exploring the Potential of High-Resolution Satellite Imagery for the Detection of Soybean Sudden Death Syndrome. Remote Sens. 12: 1213. doi: 10.3390/rs12071213
Ridenour JB, Bluhm BH (2017) The novel fungal-specific gene FUG1 has a role in pathogenicity and fumonisin biosynthesis in Fusarium verticillioides. Molecular Plant Pathology 18: 513–528. doi: 10.1111/mpp.12414
Rodriguez MC, Sautua F, Scandiani M, et al. (2021) Current recommendations and novel strategies for sustainable management of soybean sudden death syndrome. Pest Manag Sci. Accepted Author Manuscript. doi: 10.1002/ps.6458
Rosati RG, Lario LD, Hourcade ME, et al. (2018) Primary metabolism changes triggered in soybean leaves by Fusarium tucumaniae infection. Plant Science 274: 91-100. doi: 10.1016/j.plantsci.2018.05.013
Rosati RG, Ramos RS, Scandiani MM, et al. (2020) Fusarium tucumaniae and Fusarium crassistipitatum to soybean and Arabidopsis thaliana. Plant Pathology. doi: 10.1111/ppa.13286
Rosenzweig N, Tiedje JM, Quensen JF, et al. (2012) Microbial communities associated with potato common scab-suppressive soil determined by pyrosequencing analyses. Plant Dis. 96:718-725. doi: 10.1094/PDIS-07-11-0571
Roth MG, Noel ZA, Wang J, et al. (2019) Predicting Soybean Yield and Sudden Death Syndrome Development Using At-Planting Risk Factors. Phytopathology. 109(10):1710-1719. doi: 10.1094/PHYTO-02-19-0040-R
Roy KW, Rupe JC, Hershman DE, Abney TS (1997) Sudden death syndrome of soybean. Plant Dis. 81: 1100-1111. doi: 10.1094/PDIS.1997.81.10.1100
Rupe JC, Gbur EE Jr (1995) Effect of plant age, maturity group, and the environment on disease progress of sudden death syndrome of soybean. Plant Dis. 79:139-143. doi: 10.1094/PD-79-0139
Rupe JC, Robbins RT, Gbur EE (1997) Effect of crop rotation on soil population densities of Fusarium solani and Heterodera glycines and on the development of sudden death syndrome of soybean. Crop Prot. 16:575-580. doi: 10.1016/S0261-2194(97)00031-8
Rupe JC, Sabbe WE, Robbins RT, Gbur EE Jr (1993) Soil and plant factors associated with sudden death syndrome of soybean. J. Prod. Agric. 6:218-221. doi: 10.2134/jpa1993.0218
Rupe JC, Hartman GL (1999) Sudden death syndrome. In: Hartman GL, Sinclair JC, Rupe JC (Eds.) Compendium of Soybean Diseases. 4º ed. St. Paul MN. APS Press. pp. 37-39.
Ryan PR, Dessaux Y, Thomashow LS, Weller DM (2009) Rhizosphere engineering and management for sustainable agriculture. Plant Soil 321:363-383. doi: 10.1007/s11104-009-0001-6
Sagova-Mareckova M, Daniel O, Omelka M, et al. (2015) Determination of factors associated with natural soil suppressivity to potato common scab. PLoS One 10:e0116291. doi: 10.1371/journal.pone.0116291
Sahu BB, Baumbach JL, Singh P, et al. (2017) Investigation of the Fusarium virguliforme Transcriptomes Induced during Infection of Soybean Roots Suggests that Enzymes with Hydrolytic Activities Could Play a Major Role in Root Necrosis. PLoS ONE 12(1): e0169963. doi: 10.1371/journal.pone.0169963
Sang H, Witte A, Jacobs JL, et al. (2018) Fluopyram Sensitivity and Functional Characterization of SdhB in the Fusarium solani Species Complex Causing Soybean Sudden Death Syndrome. Front. Microbiol. 9: 2335. doi: 10.3389/fmicb.2018.02335
Sang H, Chang H-X, Choi S, et al. (2022) Genome-wide transcriptional response of the causal soybean sudden death syndrome pathogen Fusarium virguliforme to a succinate dehydrogenase inhibitor fluopyram. Pest Manag Sci, 78: 530-540. doi: 10.1002/ps.6657
Sanogo S, Yang XB, Scherm H (2000) Effects of Herbicides on Fusarium solani f. sp. glycines and Development of Sudden Death Syndrome in Glyphosate-Tolerant Soybean. Phytopathology. 90(1):57-66. doi: 10.1094/PHYTO.2000.90.1.57
Sanogo S, Yang XB (2001) Relation of sand content, pH, and potassium and phosphorus nutrition to the development of sudden death syndrome in soybean. Can. J. Plant Pathol. 23:174. doi: 10.1080/07060660109506927
Scandiani MM, Ruberti D, O’Donnell K, et al. (2004) Recent outbreak of soybean sudden death syndrome caused by Fusarium virguliforme and Fusarium tucumaniae in Argentina. Plant Disease 88: 1044. doi: 10.1094/PDIS.2004.88.9.1044C
Scandiani MM, Ruberti D, Giorda L, et al. (2011) Comparison of inoculation methods for characterizing relative aggressiveness of two soybean sudden-death syndrome pathogens: Fusarium virguliforme and F. tucumaniae. Tropical Plant Pathology 36:133-140.
Scandiani M, Carmona M, Luque S, et al. (2012) Isolation, identification and yield losses associated with sudden death syndrome in soybeans in Argentina. Tropical Plant Pathology 37(5): 358-362. DOI: 10.1590/S1982-56762012000500009
Scherm H, Yang XB (1999) Risk assessment for sudden death syndrome of soybean in the north-central United States. Agric. Syst. 59:301-310. doi: 10.1016/S0308-521X(99)00011-6
Scherm H, Yang XB, Lundeen P (1998) Soil variables associated with sudden death syndrome in soybean fields in Iowa. Plant Dis. 82:1152-1157. doi: 10.1094/PDIS.1998.82.10.1152
Schottel JL, Shimizu K, Kinkel LL (2001) Relationships of in vitro pathogen inhibition and soil colonization to potato scab biocontrol by antagonistic Streptomycesspp. Biol. Control 20:102-112. doi: 10.1006/bcon.2000.0893
Sharifi Tehrani A, Ramezani M (2003) Biological control of Fusarium oxysporum, the causal agent of onion wilt by antagonistic bacteria. Communications Agric. Appl. Biol. Sci. 68:543-547.
Silva LLd, Tian H, Schemerhorn B, et al. (2023) Genome-Wide Informative Microsatellite Markers and Population Structure of Fusarium virguliforme from Argentina and the USA. Journal of Fungi 9(11): 1109. doi: 10.3390/jof9111109
, , Soybean sudden death syndrome: Fungal pathogenesis and plant response. Plant Pathol 00: 1– 10. doi: 10.1111/ppa.13275
Srour AY, Fakhoury AM, Brown RL (2017) Targeting aflatoxin biosynthetic genes. Pages 159-171 in: Mycotoxigenic Fungi: Methods and Protocols. A. Moretti and A. Susca, eds. Springer, New York. doi: 10.1007/978-1-4939-6707-0_10
Srour AY, Gibson DJ, Leandro LFS, et al. (2017) Unraveling Microbial and Edaphic Factors Affecting the Development of Sudden Death Syndrome in Soybean. Phytobiomes 1(2): 91-101. doi: 10.1094/PBIOMES-02-17-0009-R
Tewoldemedhin YT, Lamprecht SC, Vaughan MM, et al. (2017) Soybean SDS in South Africa is Caused by Fusarium brasiliense and a Novel Undescribed Fusarium sp. Plant Dis. 101(1): 150-157. doi: 10.1094/PDIS-05-16-0729-RE
Turner TR, James EK, Poole PS (2013) The plant microbiome. Genome Biol. 14:209. doi: 10.1186/gb-2013-14-6-209
Vicente I, Quaratiello G, Baroncelli R, et al. (2022) Insights on KP4 Killer Toxin-like Proteins of Fusarium Species in Interspecific Interactions. Journal of Fungi. 8(9): 968. doi: 10.3390/jof8090968
Vick CM, Bond JP, Chong SK, Russin JS (2006) Response of soybean sudden death syndrome to tillage and cultivar. Can. J. Plant Pathol.-Rev. Can. Phytopathol. 28:77-83. doi: 10.1080/07060660609507273
Vilgalys R, Hester M (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 172:4238-4246. doi: 10.1128/jb.172.8.4238-4246.1990
Wang B, Swaminathan S, Bhattacharyya MK (2015) Identification of Fusarium virguliforme FvTox1-Interacting Synthetic Peptides for Enhancing Foliar Sudden Death Syndrome Resistance in Soybean. PLoS ONE 10(12): e0145156. doi: 10.1371/journal.pone.0145156
Wang J, Bradley CA, Stenzel O, et al. (2017) Baseline sensitivity of Fusarium virguliforme to fluopyram fungicide. Plant Dis., 101: 576–582. doi: 10.1094/PDIS-09-16-1250-RE
Wang B, Sumit R, Sahu BB, et al. (2018) Arabidopsis Novel Glycine-Rich Plasma Membrane PSS1 Protein Enhances Disease Resistance in Transgenic Soybean Plants. Plant Physiology 176 (1) 865-878; doi: 10.1104/pp.16.01982
Wang J, Sang H, Jacobs JL, et al. (2019) Soybean sudden death syndrome causal agent Fusarium brasiliense present in Michigan. Plant Dis., 103, 1234–1243. doi: 10.1094/PDIS-08-18-1332-RE
Wang J, Jacobs JL, Roth MG, Chilvers MI (2019) Temporal dynamics of Fusarium virguliforme colonization of soybean roots. Plant Dis., 103, 19-27. doi: 10.1094/PDIS-03-18-0384-RE
Weibelzahl-Fulton E, Dickson DW, Whitty EB (1996) Suppression of Meloidogyne incognita and M. javanica by Pasteuria penetrans in field soil. J. Nematol. 28:43-49.
Weller DM, Raaijmakers JM, Gardener BB, Thomashow LS (2002) Microbial populations responsible for specific soil suppressiveness to plant pathogens. Annu. Rev. Phytopathol. 40:309-348. doi: 10.1146/annurev.phyto.40.030402.110010
Wen Z, Tan R, Yuan J, et al. (2014) Genome-wide association mapping of quantitative resistance to sudden death syndrome in soybean. BMC Genomics 15: 809. doi: 10.1186/1471-2164-15-809
Westphal A, Becker JO (2001) Components of soil suppressiveness against Heterodera schachtii. Soil Biol. Biochem. 33:9-16. doi: 10.1016/S0038-0717(00)00108-5
Westphal A, Abney TS, Xing L, Shaner G (2008) Sudden Death Syndrome. The Plant Health Instructor. doi: 10.1094/PHI-I-2008-0102-01
Westphal A, Xing LJ (2011) Soil suppressiveness against the disease complex of the soybean cyst nematode and sudden death syndrome of soybean. Phytopathology 101:878-886. DOI: 10.1094/PHYTO-09-10-0245.
Westphal A, Li C, Xing L, et al. (2014) Contributions of Fusarium virguliforme and Heterodera glycines to the Disease Complex of Sudden Death Syndrome of Soybean. PLoS ONE 9(6): e99529. doi: 10.1371/journal.pone.0099529
Wrather A, Koenning S (2009) Effects of diseases on soybean yields in the United States 1996 to 2007. Plant Health Progress. Published online. doi: 10.1094/PHP-2009-0401-01-RS
Wrather JA, Koenning SR (2006) Estimates of disease effects on soybean yields in the United States 2003 to 2005. J. Nematol. 38:173-180.
Xing LJ, Westphal A (2006) Interaction of Fusarium solani f. sp. glycines and Heterodera glycines in sudden death syndrome of soybean. Phytopathology 96:763-770. DOI: 10.1094/PHYTO-96-0763.
Xing LJ, Westphal A (2009) Effects of crop rotation of soybean with corn on severity of sudden death syndrome and population densities of Heterodera glycines in naturally infested soil. Field Crops Res 112: 107–117. doi: 10.1016/j.fcr.2009.02.008
Xing LJ, Westphal A (2013) Synergism in the interaction of Fusarium virguliforme with Heterodera glycines in sudden death syndrome of soybean. J. Plant Dis. Prot. 120:209-217. doi: 10.1007/BF03356477
Yang S, Li X, Chen C, et al. (2016) Assessing Field-Specific Risk of Soybean Sudden Death Syndrome Using Satellite Imagery in Iowa. Phytopathology 106(8): 842-53. doi: 10.1094/PHYTO-11-15-0303-R
Zaworski Edward R (2014) Effects of ILeVO® on soybean sudden death syndrome and soybean cyst nematode. Graduate Theses and Dissertations. 14261. http://lib.dr.iastate.edu/cgi/viewcontent.cgi?article=5268&context=etd
Zhang Z, Schwartz S, Wagner L, Miller W (2000) A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 7:203-214. doi: 10.1089/10665270050081478
Zhang J, Singh A, Mueller DS, Singh AK (2015) Genome-wide association and epistasis studies unravel the genetic architecture of sudden death syndrome resistance in soybean. Plant J. 84(6): 1124-36. doi: 10.1111/tpj.13069