.
Condición fitosanitaria: Presente / Presente
Grupo de cultivos: Oleaginosas
Especie hospedante: Soja (Glycine max)
Rango de hospedantes: específico / estrecho
Etiología: Hongo. Necrotrófico
Agente causal:
Phomopsis sojae (anamorfo) / Diaporthe sojae Lehman, 1923 (teleomorfo), es el agente causal del tizón de la vaina y el tallo
Phomopsis longicolla (anamorfo) / Diaporthe longicolla Hobbs, 1985 (teleomorfo), es el agente principal de la pudrición y decaimiento de la semilla
.
Taxonomía: Eukaryota > Fungi > Dikarya > Ascomycota > Pezizomycotina > Sordariomycetes > Diaporthales > Diaporthaceae > Diaporthe
.
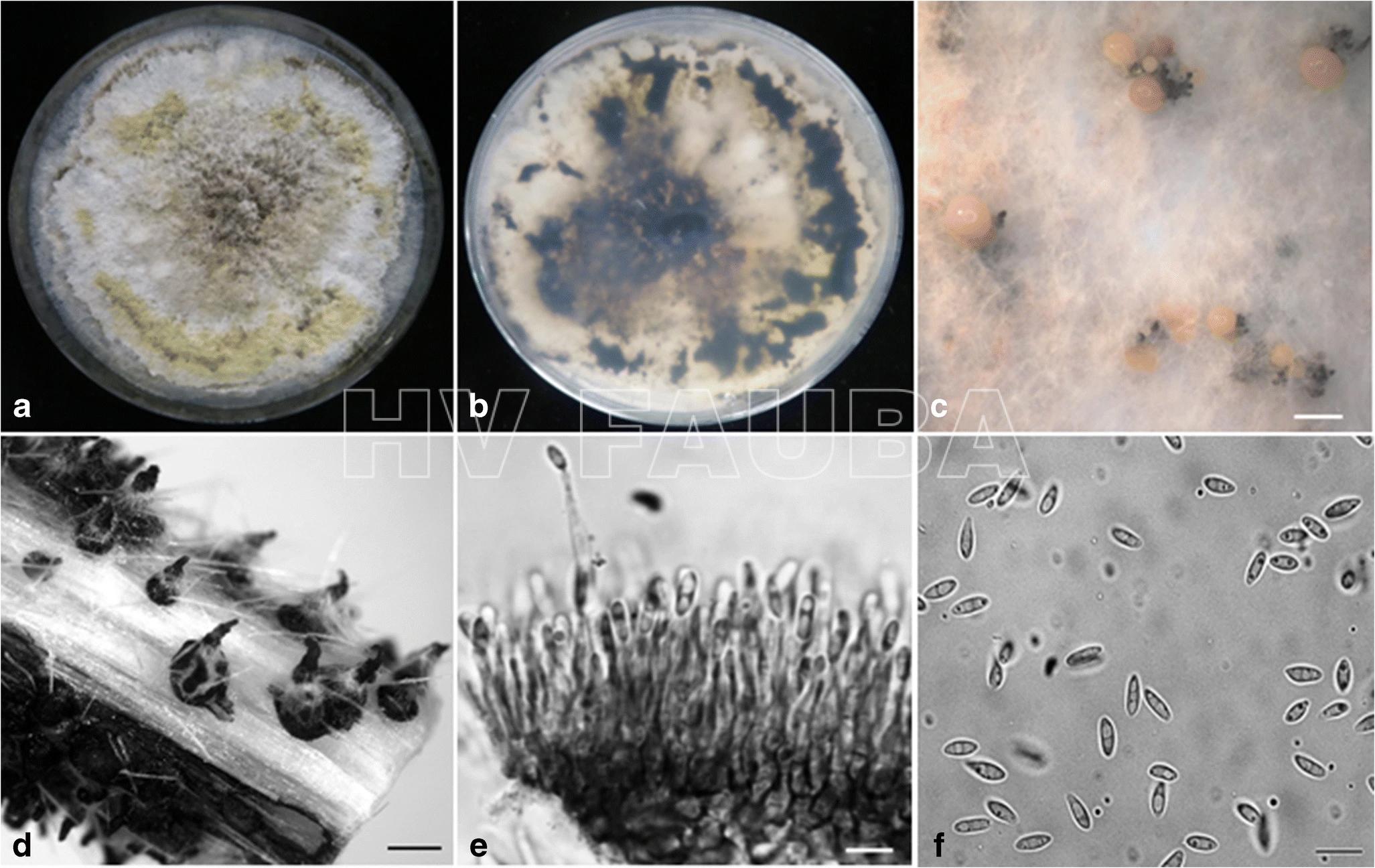
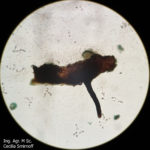
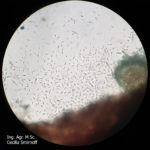
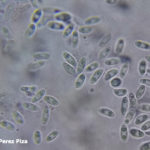
- Órganos reproductivos de Phomopsis longicolla: (a) conidiomas picnidiales; (b) estroma con picnidios maduros; (c) α conidios; (d) β conidios. Autor: Vidić et al., 2013.
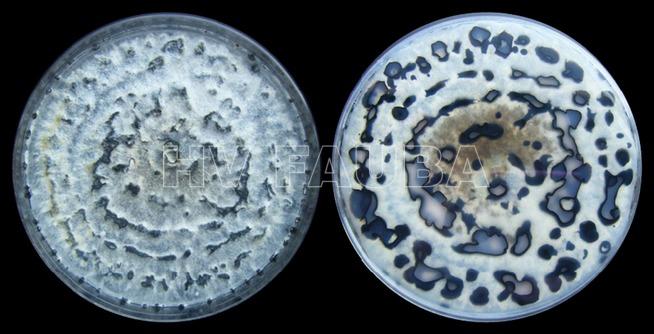
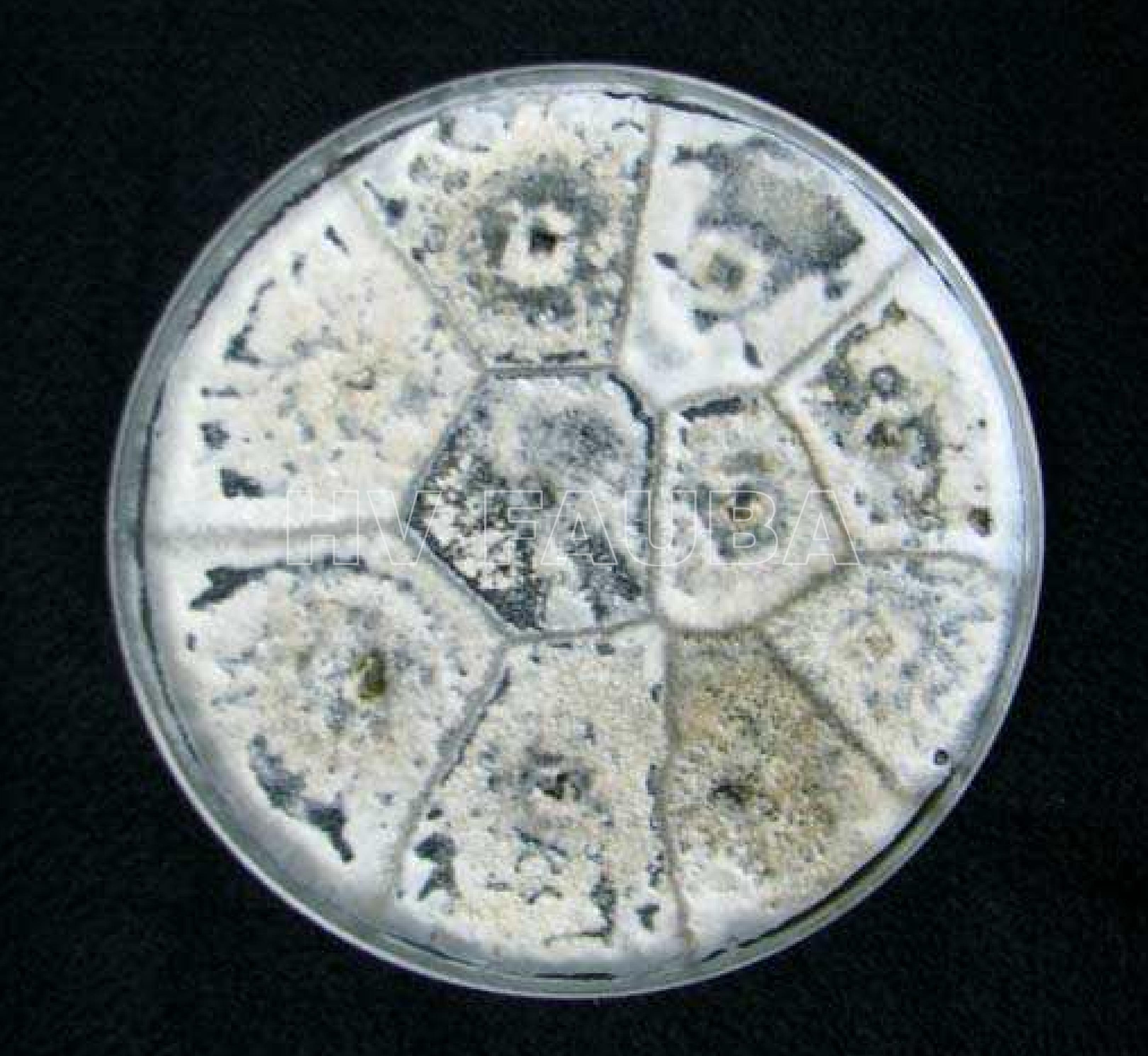
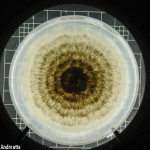
- Morfología de colonias de Phomopsis longicolla en medio APD. Autor: Vidić et al., 2013.
- Aislados de Phomopsis longicolla recolectados en el delta del Mississippi en 2006 y cultivados en agar papa dextrosa acidificado (pH 4,5) a 24°C durante 20 días. Autor: Li S, 2010.
- D. longicolla. (a) Vista superficial de los cultivos en APD acidificado después de 1 mes. (b) Vista trasera de los cultivos. (c) Conidiomas esporulados en APDA. (d) Picnidios en tallo de soja en cultivo. (e) Células conidiógenas y conidióforos. (f) α-conidios. Barras de escala (c, d) 500 μm, (e, f) 10 μm. Autor: Hosseini et al., 2020.
.
.
.
Síntomas
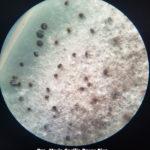
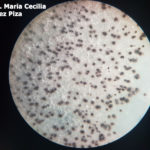
Los síntomas más frecuentes son observados en semilla, vainas y tallos. El signo característico es la presencia de picnidios (se observan como puntuaciones negras), en hileras en tallos y dispersos en vainas, los cuales bajo condiciones de alta humedad incrementan su número. La semilla infectada puede presentarse arrugada y muchas veces cubierta por micelio blanco. Sin embargo, puede tener aspecto normal y estar infectada.
.
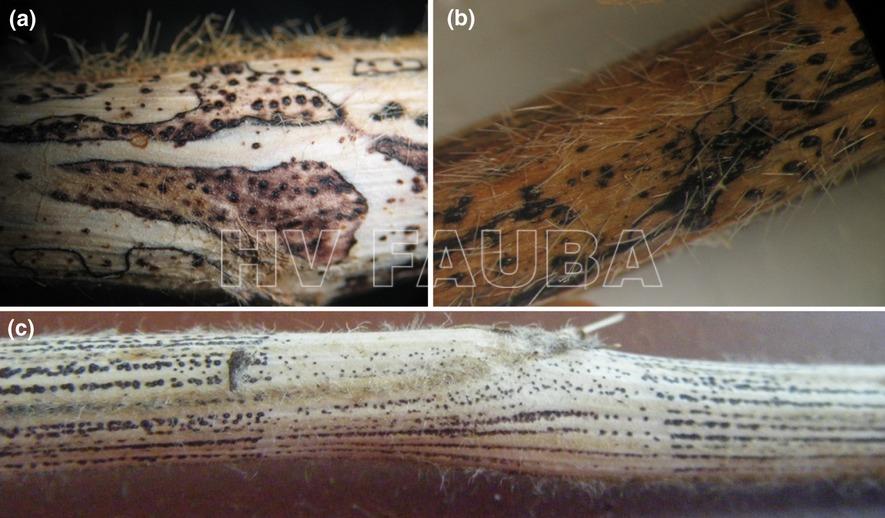
- Picnidios en tallo y vaina de soja infectados con Phomopsis. Autor: Clemson University – USDA Cooperative Extension Slide Series , Bugwood.org
- Vaina y tallos de soja con picnidios producidos por el hongo causante del tizón de la vaina y del tallo, Phomopsis. Fuente: APS Press.
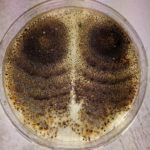
- Síntomas de Phomopsis longicolla en plantas de soja: (a) síntomas atípicos en la parte basal del tallo, infección natural; (b) síntomas atípicos en el tallo basal después de la inoculación con el micelio y conidios; (c) síntomas típicos en el tallo, infección natural. Autor: Vidić et al., 2013.
.
Condiciones ambientales predisponentes para el establecimiento de la enfermedad
Temperaturas superiores a 20 ºC y lluvias a partir de madurez fisiológica favorecen la infección de la semilla a partir de las paredes carpelares de la vaina. La lluvia está fuertemente asociada al crecimiento de esta enfermedad. La infección de semillas es más grave cuanto más se demore la cosecha en esas condiciones.
.
Importancia relativa
Alta. De gran prevalencia en todas las zonas, Phomopsis es uno de los patógenos que más disminuyen la calidad de la semilla, generando alta mortandad de plántulas y disminuyendo el poder germinativo.
.
- La pudrición de la semilla de Phomopsis es causada principalmente por el hongo Phomopsis longicolla. Los síntomas y signos se manifiestan en vainas y semillas de plantas en ambientes cálidos y húmedos. Autor: Li S, 2010.
- Autor: Dra. Maria Cecilia Perez Piza
.
Manejo de la enfermedad
Sembrar semillas sanas o tratadas con fungicidas. Fertilizar adecuadamente los lotes. Rotación de cultivos. Aplicación de fungicidas foliares.
.
- Picnidio de Phomopsis spp. con cirro sobre tallo de soja. Autor: Dr. Francisco Sautua
- Picnidio de Phomopsis spp. con cirro sobre tallo de soja. Autor: Dr. Francisco Sautua
- Peritecio de Diaporthe spp
- Ascosporas de Diaporthe spp
- Autor: Dra. Maria Cecilia Perez Piza
- Autor: Dra. Maria Cecilia Perez Piza
- Autor: Dra. Maria Cecilia Perez Piza
- Autor: Dra. Maria Cecilia Perez Piza
- Autor: Dra. Maria Cecilia Perez Piza
- Autor: Dra. Maria Cecilia Perez Piza
- Autor: Dra. Maria Cecilia Perez Piza
- Autor: Dra. Maria Cecilia Perez Piza
- Autor: Dra. Maria Cecilia Perez Piza
- Autor: Dra. Maria Cecilia Perez Piza
- Autor: Dra. Maria Cecilia Perez Piza
- Autor: Dra. Maria Cecilia Perez Piza
- Autor: Dra. Maria Cecilia Perez Piza
.
.
.
Bibliografía
Athow KL, Caldwell RM (1954) A comparative study of Diaporthe stem canker and pod stem blight of soybean. Phytopathology 44: 319–325.
Baird RE, Abney TS, Mullinix BG (2001) Fungi associated with pods and seeds during the R6 and R8 stages of four soybean cultivars in southwestern Indiana. Phytoprotection 82: 1–11. doi: 10.7202/706210ar
Brumer BB, Lopes-Caitar VS, Chicowski AS, et al. (2018) Morphological and molecular characterization of Diaporthe (anamorph Phomopsis) complex and pathogenicity of Diaporthe aspalathi isolates causing stem canker in soybean. Eur J Plant Pathol 151: 1009–1025. doi: 10.1007/s10658-018-1436-5
Costamilan LM, Yorinori JT, Almeida AMR, et al. (2008) First report of Diaporthe phaseolorum var. caulivora infecting soybean plants in Brazil. Trop Plant Pathol 33: 381–385. doi: 10.1590/S1982-56762008000500007
Fernández FA, Hanlin RT (1996) Morphological and RAPD analyses of Diaporthe phaseolorum from soybean. Mycologia 88: 425–440. doi: 10.1080/00275514.1996.12026670
Floyd CM, Malvick DK (2022) Diaporthe species associated with symptomatic and asymptomatic infection of soybean stems in Minnesota: identity, virulence, and growth characteristics. Canadian Journal of Plant Pathology 44: 858-873. doi: 10.1080/07060661.2022.2077450
Ghimire K, Petrović K, Kontz BJ, et al. (2019) Inoculation Method Impacts Symptom Development Associated with Diaporthe aspalathi, D. caulivora, and D. longicolla on Soybean (Glycine max). Plant Dis. 103(4): 677-684. doi: 10.1094/PDIS-06-18-1078-RE
Gomes RR, Glienke C, Videira SIR, et al. (2013) Diaporthe: a genus of endophytic, saprobic and plant pathogenic fungi. Persoonia 31: 1–41. doi: 10.3767/003158513X666844
Grau CR, Oplinger ES (1981) Influence of cultural practices and foliar fungicides on soybean yield, Phomopsis seed infection and germination. Proceedings Soybean Seed Research Conference, vol. 11, pp. 1–8.
Grijalba P, Ridao AC (2012) Survival of Diaporthe phaseolorum var. caulivora (causal agent of soybean stem canker) artificially inoculated in different crop residues. Trop Plant Pathol 37(4): 271–274. doi: 10.1590/S1982-56762012000400006
Gupta A., Kumar R. (2020) Management of Seed-Borne Diseases: An Integrated Approach. In: Kumar R., Gupta A. (eds) Seed-Borne Diseases of Agricultural Crops: Detection, Diagnosis & Management. Springer, Singapore. doi: 10.1007/978-981-32-9046-4_25
Hobbs TW, Schmitthenner AF, Kuter GA (1985) A new Phomopsis species from soybean. Mycologia 77: 534-544. doi: 10.1080/00275514.1985.12025139
Hosseini B, El-Hasan A, Link T, et al. (2020) Analysis of the species spectrum of the Diaporthe/Phomopsis complex in European soybean seeds. Mycol Progress 19: 455–469. doi: 10.1007/s11557-020-01570-y
Kmetz KT, Schmitthenner AF, Ellett CW (1978) Soybean seed decay: prevalence of infection and symptom expression caused by Phomopsis sp., Diaporthe phaseolorum var. sojae, and D. phaseolorum var. caulivora. Phytopathology 68: 836–840. doi: 10.1094/Phyto-68-836
Ku Y-S, Cheng S-S, Gerhardt A, Cheung M-Y, Contador CA, Poon L-YW, Lam H-M (2020) Secretory Peptides as Bullets: Effector Peptides from Pathogens against Antimicrobial Peptides from Soybean. International Journal of Molecular Sciences 21(23): 9294. doi: 10.3390/ijms21239294
Kulik MM (1984) Symptomless infection, persistence, and production of pycnidia in host and non-host plants by Phomopsis batatae, Phomopsis phaseoli, and Phomopsis sojae, and the taxonomic implications. Mycologia 76(2): 274–291. doi: 10.1080/00275514.1984.12023837
Lehman SG (1923) Pod and stem blight of soybean. Ann Mo Bot Gard 10: 111–178.
Li S (2010) Phomopsis Seed Decay of Soybean. Soybean – Molecular Aspects of Breeding. IntechOpen. doi: 10.5772/15688
Li S, Chen P (2013) Resistance to Phomopsis Seed Decay in Soybean. ISRN Agronomy Article ID 738379. doi: 10.1155/2013/738379
Li S, Darwish O, Alkharouf NW, et al. (2017) Analysis of the genome sequence of Phomopsis longicolla: a fungal pathogen causing Phomopsis seed decay in soybean. BMC Genomics 18(1):688. doi: 10.1186/s12864-017-4075-x
Li S, Deng Y (2021) Mitochondrial Genome Resource of Phomopsis longicolla, a Fungus Causing Phomopsis Seed Decay in Soybean. PhytoFrontiers™ 0 0:0. doi: 10.1094/PHYTOFR-10-20-0027-A
Mengistu A, Heatherly LG (2006) Planting date, irrigation, maturity group, year, and environment effects on Phomopsis longicolla, seed germination, and seed health rating of soybean in the early soybean production system of the midsouthern USA. Crop Protection 25: 310-317. doi: 10.1016/j.cropro.2005.05.011
Mengistu A, Castlebury LA, Smith JR, et al. (2009) Seasonal progress of Phomopsis longicolla infection on soybean plant parts and its relationship to seed quality. Plant Dis. 93: 1009-1018. doi: 10.1094/PDIS-93-10-1009
Mengistu A, Castlebury LA, Morel W, et al. (2014) Pathogenicity of Diaporthe spp. isolates recovered from soybean (Glycine max) seeds in Paraguay. Can J Plant Pathol 36(4): 470–474. doi: 10.1080/07060661.2014.966151
Morgen-Jones G (1985) The Diaporthe/Phomopsis complex of soybean: morphology. In: Proceedings of the conference on Diaporthe/Phomopsis disease complex of soybean. United States Department of Agriculture, Beltsville, pp 1–7
Morgan-Jones G (1989) The Diaporthe/Phomopsis complex: taxonomic considerations. In: Pascale A (ed) Proceedings of the world soybean research conference IV. Orientación Gráfica, Buenos Aires, pp 1699–1706.
Morris TC, Vann RA, Collins GD, et al. (2021) Planting date and maturity group impact on soybean seed quality in the southeastern United States. Agronomy Journal. 113: 5287– 5301. doi: 10.1002/agj2.20913
Nevena M, Jelena V, Franić-Mihajlović D (1997) A comparative study of Diaporthe/Phomopsis fungi on soybean from two different regions of the world. Mycopathologia 139: 107–113. doi: 10.1023/A:1006849020432
Petrović K, Vidić M, Riccioni L, et al. (2015) First report of Diaporthe eres species complex causing seed decay of soybean in Serbia. Plant Dis 99(8): 1186. doi: 10.1094/PDIS-01-15-0056-PDN
Petrovic K, Skaltsas D, Castlebury L, et al. (2020) Diaporthe seed decay of soybean [Glycine max (L.) Merr.] is endemic in the United States, but new fungi are involved. Plant Disease. doi: 10.1094/PDIS-03-20-0604-RE
Petrović K, Skaltsas D, Castlebury LA, et al. (2021) Diaporthe Seed Decay of Soybean [Glycine max (L.) Merr.] Is Endemic in the United States, But New Fungi Are Involved. Plant Dis. 105(6): 1621-1629. doi: 10.1094/PDIS-03-20-0604-RE
Petrović K, Šućur Elez J, Crnković M, et al. (2023) The Biochemical Response of Soybean Cultivars Infected by Diaporthe Species Complex. Plants 12(16): 2896. doi: 10.3390/plants12162896
Pioli RN, Morandi EN, Biasaro V (2001) First report of soybean stem canker caused by Diaporthe phaseolorum var. caulivora in Argentina. Plant Dis 85: 95. doi: 10.1094/PDIS.2001.85.1.95B
Rossman AY, Adams GC, Cannon PF, et al. (2015) Recommendations of generic names in Diaporthales competing for protection or use. IMA Fungus 6(1): 145–154. doi: 10.5598/imafungus.2015.06.01.09
Roy KW, Keith BC, Andrews CH (1994) Resistance of hardseeded soybean lines to seed infection by Phomopsis, other fungi, and soybean mosaic virus. Can. J. Plant Pathol. 16: 122-128. doi: 10.1080/07060669409500769
Santos JM, Vrandečić K, Ćosić J, et al. (2011) Resolving the Diaporthe species occurring on soybean in Croatia. Persoonia 27: 9–19. doi: 10.3767/003158511X603719
Shortt BJ, Grybauskas AP, Tenne FD, Sinclair JB (1981) Epidemiology of Phomopsis seed decay of soybean in Illinois. Plant Dis. 65: 62-64. doi: 10.1094/PD-65-62
Sinclair JB (1992) Discoloration of soybean seeds in an indicator of quality. Plant Dis 76: 1087–1091.
Sinclair JB (1993) Phomopsis seed decay of soybeans – a prototype for studying seed disease. Plant Dis 77(4): 329–334. doi: 10.1094/PD-77-0329
Sinclair JB (1999) Diaporthe–Phomopsis complex. In: Hartman GL, Sinclair JB, Rupe JC (Eds.), Compendium of Soybean Diseases (4th ed), American Phytopathological Society Press, St. Paul, MN, pp. 31-32.
Sun S, Kim MY, Chaisan T, et al. (2013) Phomopsis (Diaporthe) species as the cause of soybean seed decay in Korea. J Phytopathol 161: 131–134. doi: 10.1111/jph.12034
Tekrony DM, Egli DB, Stuckey RE, Balles J (1983) Relationship between weather and soybean seed infection by Phomopsis sp. Phytopathology 73: 914-918. doi: 10.1094/Phyto-73-914
TeKrony DM, Egli DB, Balles J, et al. (1984) Effect of date of harvest maturity on soybean seed quality and Phomopsis sp. and infection. Crop Sci., 24: 189-193. doi: 10.2135/cropsci1984.0011183X002400010045x
TeKrony DM, Grabau LJ, Delacy M, Kane M (1996) Early planting of early maturing soybean: effect on seed germination and Phomopsis infection. Agronomy J., 88: 428-433. doi: 10.2134/agronj1996.00021962008800030011x
Thomison PR, Kenworthy WJ, McIntosh MS (1990) Phomopsis seed decay in soybean isolines differing in stem termination, time of flowering, and maturity. Crop Sci. 30: 183-188. doi: 10.2135/cropsci1990.0011183X003000010040x
Udayanga D, Liu X, McKenzie EHC, et al. (2011) The genus Phomopsis: biology, applications, species concepts and names of common phytopathogens. Fungal Diversity 50: 189. doi: 10.1007/s13225-011-0126-9
Udayanga D, Liu X, Crous PW, et al. (2012) A multi-locus phylogenetic evaluation of Diaporthe (Phomopsis). Fungal Divers 56: 157–171. doi: 10.1007/s13225-012-0190-9
Udayanga D, Castlebury LA, Rossman AY, et al. (2014) Insights into the genus Diaporthe: phylogenetic species delimitation in the D. eres species complex. Fungal Divers 67: 203–229. doi: 10.1007/s13225-014-0297-2
Udayanga D, Castlebury LA, Rossman AY, Hyde KD (2014) Species limits in Diaporthe: molecular re-assessment of D. citri, D. cytosporella, D. foeniculina and D. rudis. Persoonia 32: 83–101. doi: 10.3767/003158514X679984
Vidić M, Petrović K, Đorđević V, Riccioni L (2013) Occurrence of Phomopsis longicolla β Conidia in Naturally Infected Soybean. J Phytopathol 161: 470-477. doi: 10.1111/jph.12092
Walcott RR (2014) Acidified PDA method for the detection of Phomopsis complex on Glycine max. In: International rules for seed testing. International Seed Testing Association (ISTA), Bassersdorf, pp 7–016.
Wrather JA, Kendig SR, Wiebold WJ, Riggs RD (1996) Cultivar and planting date effects on soybean stand, yield, and Phomopsis sp. seed infection. Plant Disease 80: 622-624. doi: 10.1094/PD-80-0622
Zhang AW, Riccioni L, Pedersen WL, et al. (1998) Molecular identification and phylogenetic grouping of Diaporthe phaseolorum and Phomopsis longicolla isolates from soybean. Phytopathology 88(12): 1306–1314. doi: 10.1094/PHYTO.1998.88.12.1306