.
Condición fitosanitaria: Presente
Grupo de cultivo: Oleaginosas
Especie Hospedante: Glycine max
Rango de hospedantes: amplio / no específico
Epidemiología: monocíclica, aguda.
Etiología: Pseudo-fungi Necrotrófico
Agente Causal: Pythium spp. (*)
Taxonomia: Eukaryota > Stramenopiles > Oomycota > Pythiales > Pythiaceae > Pythium
.
Hasta la fecha, se han identificado más de 354 especies de Pythium (Jiang et al., 2012; van der Plaats-Niterink, 1981). Relevamientos del norte y sur de la provincia de Buenos Aires, han identificado 13 especies de Pythium, predominando P. irregulare y P. ultimum en ambas regiones (Grijalba et al., 2021).
.
- Damping off post emergencia. Autor: Dr. Pablo Grijalba
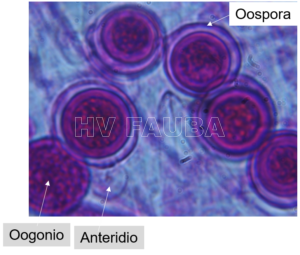
- Oogonio y anteridio de P. ultimun. Autor: Dr. Pablo Grijalba
- Oosporas. Autor: Dr. Pablo Grijalba
.
.
.
Antecedentes
El “damping off” y la podredumbre de raíces por Pythium spp es una enfermedad de plántulas común en todas las regiones sojeras de la Argentina.
.
Sintomatología
Podredumbre de la semilla, y muerte de plántulas o “damping off” de pre-emergencia, que se evidencia como falta de plantas en el lote, y también puede provocar “damping off” de post-emergencia. Algunas plantas, bajo condiciones de crecimiento más favorables, continúan su desarrollo a pesar de la presencia de raíces infectadas, pero aunque las plantas sobrevivan a la infección inicial, el patógeno reduce el vigor y el rendimiento de las mismas, especialmente en condiciones de estrés. El nivel de infección decrece con la edad de las plántulas. La infección ocurre generalmente en suelos muy húmedos, donde la concentración de oxigeno es baja, lo que estimula que los exudados radiculares sean más ricos en azúcares y aminoácidos, lo que favorece la dirección de los propágulos hacia las raíces. Se ha observado que el damping off aumenta con la siembra directa, debido principalmente al aumento de la humedad y a la disminución de la temperatura del suelo, y muchas veces también al aumento del inóculo inicial.
.
Agente causal
De acuerdo con relevamientos efectuados por la cátedra de Fitopatología FAUBA, son muchas las especies del género Pythium que atacan al cultivo de soja, entre ellas se pueden citar para nuestro país a: P. ultimun, P. irregulare, P. sylvaticum, P. aphanidermathum, entre otros, siendo las dos primeras las más prevalentes.
.
Condiciones ambientales predisponentes
El patógeno presenta zoosporangios que germinan directamente o por medio de zoosporas ciliadas. Por esto último la presencia de agua libre en el suelo constituye un factor de riesgo. Como esporas sexuales presentan oosporas, las que pueden permanecer en el suelo varios años, pero como es un habitante de suelo puede vivir saprofíticamente en los rastrojos. Actualmente, con grupos de madurez cortos, se está intentando sembrar lo más temprano posible. Pero el sembrar en suelos fríos por debajo de 15 ºC, esto también constituye un factor de riesgo, ya que los ataque ocasionados por P. ultimum son más graves a temperaturas más bajas (por debajo de 20 °C), si bien hay especies que se desarrollan a temperaturas óptimas algo mayores (25-30 ºC).
.
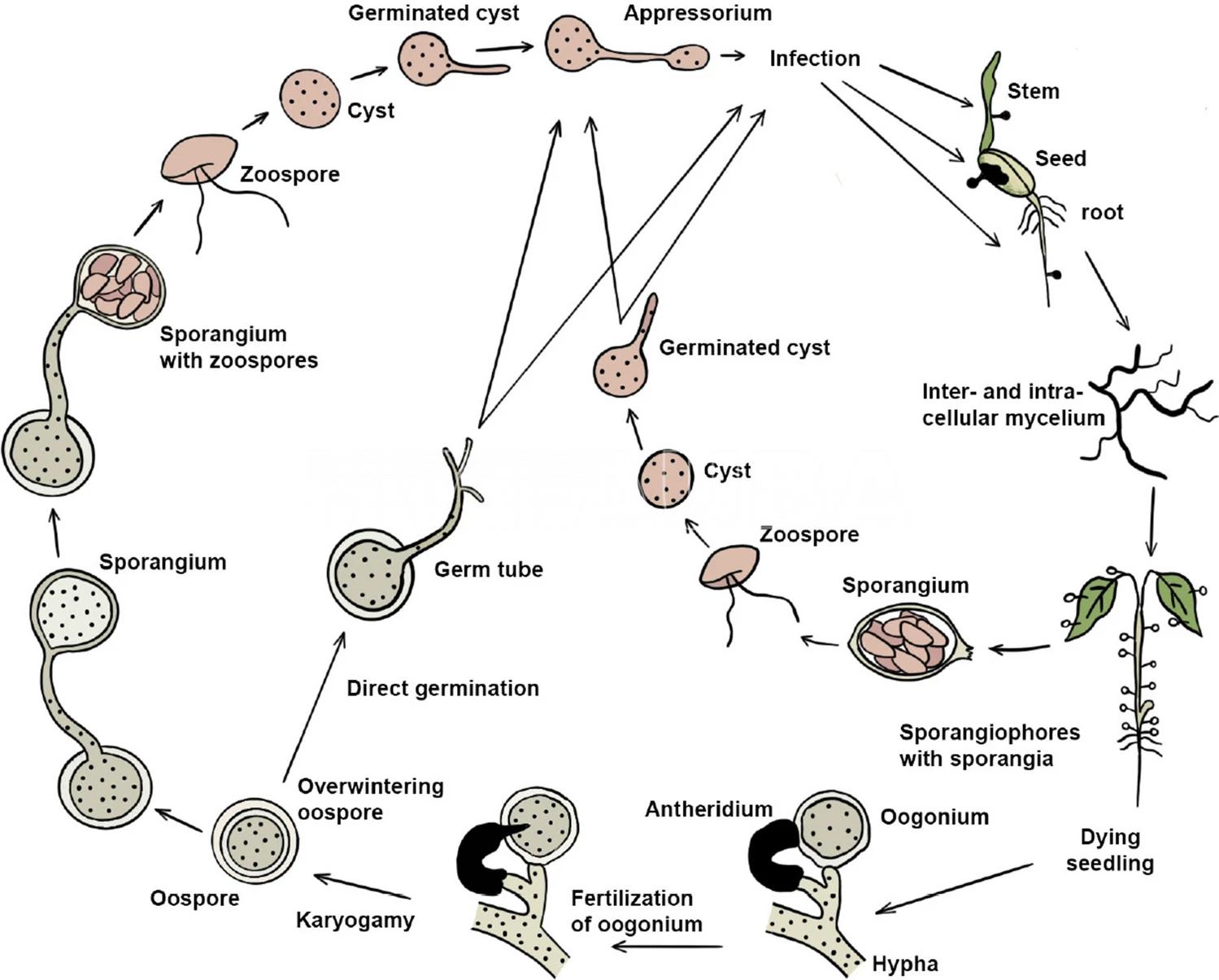
- Ciclo biológico de Pythium spp. El ciclo agronómico se considera monocíclico. Autores: West et al. 2003; Jayawardena et al., 2020.
.
Manejo de la enfermedad
Si bien hay bibliografía que indica algún tipo de resistencia a Pythium aphanidermatum, hasta el presente no hay resistencia genética disponible para este género y se deben implementar distintos tipos de medidas dentro de un manejo integrado de la enfermedad. Entre las que se pueden citar:
1) Mejorar el drenaje del suelo principalmente en un sistema de siembra directa,
2) Evitar el riego durante los primeros 10 días después de la siembra. Cuanto antes se produzca la emergencia de la semilla menos posibilidades tendrá de sufrir ataques de Pythium, por lo que:
3) sembrar en suelos cálidos, por encima de 18 ºC es altamente recomendable.
4) La principal medida es el tratamiento de la semilla con fungicidas específicos para Pythiaceas.
.
.
.
Bibliografía
Bates GD, Rothrock CS, Rupe JC (2008) Resistance of the soybean cultivar Archer to Pythium damping-off and root rot caused by several Pythium spp. Plant Disease 92: 763-766. doi: 10.1094/PDIS-92-5-0763
Broders KD, Lipps PE, Paul PA, Dorrance AE (2007) Characterization of Pythium spp. associated with corn and soybean seed and seedling disease in Ohio. Plant Disease 91(6): 727-735. doi: 10.1094/PDIS-91-6-0727
Carmona MA, Sautua FJ, Grijalba PE, Cassina M, Pérez‐Hernández O (2018) Effect of potassium and manganese phosphites in the control of Pythium damping‐off in soybean: a feasible alternative to fungicide seed treatments. Pest Management Science 74: 366-374. doi: 10.1002/ps.4714
Chi SI, Akuma M, Xu R, et al. (2024) Phenazines are involved in the antagonism of a novel subspecies of Pseudomonas chlororaphis strain S1Bt23 against Pythium ultimum. Sci Rep 14, 20517. doi: 10.1038/s41598-024-71418-y
Clevinger EM, Biyashev R, Lerch-Olson E, Yu H, Quigley C, Song Q, Dorrance AE, Robertson AE, Saghai Maroof MA (2021) Identification of Quantitative Disease Resistance Loci Toward Four Pythium Species in Soybean. Front Plant Sci. 12: 644746. doi: 10.3389/fpls.2021.644746
de Cock AW, Lodhi AM, Rintoul TL, Bala K, Robideau GP, Abad ZG, Coffey MD, Shahzad S, Lévesque CA (2015) Phytopythium: molecular phylogeny and systematics. Persoonia 34: 25-39. doi: 10.3767/003158515X685382
Delgado-Baquerizo, M., Guerra, C.A., Cano-Díaz, C. et al. (2020) The proportion of soil-borne pathogens increases with warming at the global scale. Nat. Clim. Chang. 10, 550–554. doi: 10.1038/s41558-020-0759-3
Dorrance AE, Berry SA, Lipps PE (2004) Characterization of Pythium spp. from Three Ohio Fields for Pathogenicity on Corn and Soybean and Metalaxyl Sensitivity. Plant Health Progress 5:1. doi: 10.1094/PHP-2004-0202-01-RS
Dorrance AE, Vargas A, Navarro-Acevedo K, et al. (2024) Picarbutrazox effectiveness added to a seed treatment mixture for management of Oomycetes that impact soybean in Ohio. Plant Dis. doi: 10.1094/PDIS-06-23-1223-RE
Eggertson QA, Rintoul TL, Lévesque CA (2023) Resolving the Globisporangium ultimum (Pythium ultimum) species complex. Mycologia: 1-19. doi: 10.1080/00275514.2023.2241980
, , , et al. (2020) Pathogenicity and fungicide sensitivity of Pythium and Phytopythium spp. associated with soybean in the Huang‐Huai region of China. Plant Pathology 69: 1083– 1092. doi: 10.1111/ppa.13187
Ferrarini E, De Roo V, Geudens N, et al. (2022) Altering in vivo membrane sterol composition affects the activity of the cyclic lipopeptides tolaasin and sessilin against Pythium. Biochim Biophys Acta Biomembr.: 184008. doi: 10.1016/j.bbamem.2022.184008
Griffin GJ (1990) Importance of Pythium ultimum in a disease syndrome of cv. Essex Soybean. Canadian Journal of Plant Pathology 12(2): 135-140. doi: 10.1080/07060669009501016
Grijalba PE, Ridao Ad, Steciow MM (2021) Oomycetes species associated with soybean in Buenos Aires Province (Argentina). Phytoparasitica. doi: 10.1007/s12600-021-00921-z
Harvey PR, Warren RA, Wakelin S (2008) The Pythium–Fusarium root disease complex – an emerging constraint to irrigated maize in southern New South Wales. Australian Journal of Experimental Agriculture 48(3): 367-374. doi: 10.1071/EA06091
Hebb L, Bradley CA, Telenko DEP, et al. (2023) Isolates of Phytophthora sansomeana Display a Range of Aggressiveness on Soybean Seedlings. Plant Health Progress. doi: 10.1094/PHP-08-22-0075-RS
Hudge BV (2015) Management of damping-off disease of soybean caused by Pythium ultimum Trow. Int.J.Curr.Microbiol.App.Sci 4(1): 799-808. ISSN: 2319-7706
Jayawardena RS, Hyde KD, Chen YJ, et al. (2020) One stop shop IV: taxonomic update with molecular phylogeny for important phytopathogenic genera: 76–100. Fungal Diversity 103: 87–218 (2020). doi: 10.1007/s13225-020-00460-8
Jiang YN, Haudenshield JS, Hartman GL (2012) Characterization of Pythium spp. from soil samples in Illinois. Canadian Journal of Plant Pathology 34(3): 448-454. doi: 10.1080/07060661.2012.705326
Jose A, Anitha Sasidharan S, Chacko C, et al. (2021) Activity of Clove Oil and Chitosan Nanoparticles Incorporated PVA Nanocomposite Against Pythium aphanidermatum. Appl Biochem Biotechnol. doi: 10.1007/s12010-021-03709-3
Kasteel M, Ketelaar T, Govers F (2023) Fatal attraction: How Phytophthora zoospores find their host. Semin Cell Dev Biol. 148-149: 13-21. doi: 10.1016/j.semcdb.2023.01.014
Kato M, Minamida K, Tojo M, Kokuryu T, Hamaguchi H, Shimada S (2013) Association of Pythium and Phytophthora with Pre-emergence Seedling Damping-off of Soybean Grown in a FieldConverted from a Paddy Field in Japan. Plant Production Science 16: 95-104. doi: 10.1626/pps.16.95
Kurm V, Visser J, Schilder M, et al. (2023) Soil Suppressiveness Against Pythium ultimum and Rhizoctonia solani in Two Land Management Systems and Eleven Soil Health Treatments. Microb Ecol 86: 1709–1724. doi: 10.1007/s00248-023-02215-9
Legrifi I, Al Figuigui J, El Hamss H, et al. (2022) Potential for Biological Control of Pythium schmitthenneri Root Rot Disease of Olive Trees (Olea europaea L.) by Antagonistic Bacteria. Microorganisms 10(8): 1635. doi: 10.3390/microorganisms10081635
Lévesque CA, Brouwer H, Cano L, et al. (2010) Genome sequence of the necrotrophic plant pathogen Pythium ultimum reveals original pathogenicity mechanisms and effector repertoire. Genome Biol 11: R73. doi: 10.1186/gb-2010-11-7-r73
Lin F, Li W, McCoy AG, et al. (2022) Identification and characterization of pleiotropic and epistatic QDRL conferring partial resistance to Pythium irregulare and P. sylvaticum in soybean. Theor Appl Genet. doi: 10.1007/s00122-022-04201-0
Liu Y, He P, He P, et al. (2022) Potential biocontrol efficiency of Trichoderma species against oomycete pathogens. Front Microbiol. 13: 974024. doi: 10.3389/fmicb.2022.974024
Lookabaugh EC, Kerns JP, Cubeta MA, Shew BB (2018) Fitness Attributes of Pythium aphanidermatum with Dual Resistance to Mefenoxam and Fenamidone. Plant Dis. 102(10): 1938-1943. doi: 10.1094/PDIS-01-18-0043-RE
Mahmoud MA, Omar AF, Mohamed AAA, et al. (2021) Damping-off caused by Pythium aphanidermatum on sugar beet in Egypt. Australasian Plant Dis. Notes 16: 25. doi: 10.1007/s13314-021-00438-8
Marchand G, Chen Y, Berhane NA, Wei L, Lévesque CA, Xue AG (2014) Identification of Pythium spp. from the rhizosphere of soybeans in Ontario, Canada. Canadian Journal of Plant Pathology 36(2): 246-251. doi: 10.1080/07060661.2014.920921
Matthiesen RL, Ahmad AA, Robertson AE (2016) Temperature affects aggressiveness and fungicide sensitivity of four Pythium spp. that cause soybean and corn damping off in Iowa. Plant Disease 100(3): 583-591. doi: 10.1094/PDIS-04-15-0487-RE
Matthiesen RL, Robertson AE (2023) Effect of infection timing by four Pythium spp. on soybean damping-off symptoms with and without cold stress. Plant Dis. doi: 10.1094/PDIS-01-23-0082-RE
Miao J, Liu X, Du X, Li G, Li C, Zhao D, Liu X (2020) Sensitivity of Pythium spp. and Phytopythium spp. and tolerance mechanism of Pythium spp. to oxathiapiprolin. Pest Management Science 76: 3975-3981. https://doi.org/10.1002/ps.5946
Molin C, Ribeiro NR, Matsumoto MN, et al. (2021) Damping‐off of soybean in southern Brazil can be associated with different species of Globisporangium spp. and Pythium spp. Plant Pathology 70: 1686-1694. doi: 10.1111/ppa.13397
Navarro-Acevedo KA, Wijeratne S, Culman SW, et al. (2021) The Effect of Incubation Temperature on the Species Composition of Phytophthora, Phytopythium, and Pythium Communities Associated with Soybean. Phytobiomes Journal 5: 133-144. doi: 10.1094/PBIOMES-01-20-0016-R
Navarro KA, Wijeratne S, Culman S, et al. (2021) Comparison of the Species Communities of Phytophthora, Pythium, and Phytopythium Associated with Soybean Genotypes in High Disease Environments in Ohio. Phytobiomes Journal 5: 288-304. doi: 10.1094/PBIOMES-12-20-0089-R
Navi SS, Huynh T, Mayers CG, et al. (2019) Diversity of Pythium spp. associated with soybean damping-off, and management implications by using foliar fungicides as seed treatments. Phytopathology Research 1: 8. https://doi.org/10.1186/s42483-019-0015-9
Nguyen HDT, Dodge A, Dadej K, et al. (2022) Whole genome sequencing and phylogenomic analysis show support for the splitting of genus Pythium. Mycologia 6:1-15. doi: 10.1080/00275514.2022.2045116
Panth M, Hassler SC, Baysal-Gurel F (2020) Methods for Management of Soilborne Diseases in Crop Production. Agriculture 10: 16. doi: 10.3390/agriculture10010016
Pimentel MF, Arnao E, Warner AJ, et al. (2022) Reduction of Pythium Damping-off in Soybean by Biocontrol Seed Treatment. Plant Disease. doi: 10.1094/PDIS-06-21-1313-RE
Radmer L, Anderson G, Malvick DM, et al. (2017) Pythium, Phytophthora, and Phytopythium spp. Associated with Soybean in Minnesota, Their Relative Aggressiveness on Soybean and Corn, and Their Sensitivity to Seed Treatment Fungicides. Plant Disease 101(1): 62-72. doi: 10.1094/PDIS-02-16-0196-RE
Raftoyannis Y, Dick MW (2006) Zoospore encystment and pathogenicity of Phytophthora and Pythium species on plant roots. Microbiological Research 161(1): 1-8. doi: 10.1016/j.micres.2005.04.003
Ravi A, Das S, Sebastian SK. et al. (2023) Bioactive Metabolites of Serratia sp. NhPB1 Isolated from Pitcher of Nepenthes and its Application to Control Pythium aphanidermatum. Probiotics & Antimicro. Prot. doi: 10.1007/s12602-023-10154-7
Roberts DP, Selmer K, Lupitskyy R, et al. (2021) Seed treatment with prodigiosin controls damping-off of cucumber caused by Pythium ultimum. AMB Express 11(1): 10. doi: 10.1186/s13568-020-01169-2
Rojas AJ, Jacobs JL, Napieralski S, et al. (2017) Oomycete Species Associated with Soybean Seedlings in North America—Part I: Identification and Pathogenicity Characterization. Phytopathology 107(3): 280-292. doi: 10.1094/PHYTO-04-16-0177-R
Rojas A, Jacobs JL, Napieralski S, et al. (2017) Oomycete species associated with soybean seedlings in North America – Part II: Diversity and Ecology in Relation to Environmental and Edaphic Factors. Phytopathology 107: 293–304. doi: 10.1094/PHYTO-04-16-0176-R
Rupe JC, Rothrock CS, Bates G, et al. (2011) Resistance to Pythium Seedling Disease in Soybean. pp 261- In: Sudaric A (Ed.) Soybean – Molecular Aspects of Breeding. InTech. doi: 10.5772/15301
Sartori FF, Pimpinato FR, Tornisielo VL, et al. (2020) Soybean seed treatment: how do fungicides translocate in plants?. Pest Management Science 76: 2355-2359. doi: 10.1002/ps.5771
Scott K, Eyre M, McDuffee D, Dorrance AE (2020) The Efficacy of Ethaboxam as a Soybean Seed Treatment Toward Phytophthora, Phytopythium, and Pythium in Ohio. Plant Disease 104(5): 1421-1432. doi: 10.1094/PDIS-09-19-1818-RE
Serrano M, McDuffee D, Robertson AE (2018) Damping-off caused by Pythium sylvaticum on soybeans subjected to periods of cold stress is reduced by seed treatments. Canadian Journal of Plant Pathology. doi: 10.1080/07060661.2018.1522516
Siebatcheu EC, Wetadieu D, Youassi Youassi O, et al. (2022) Secondary metabolites from an endophytic fungus Trichoderma erinaceum with antimicrobial activity towards Pythium ultimum. Nat Prod Res. 1-6. doi: 10.1080/14786419.2022.2075360
Tu CK, Wang PH, Lee MH (2022) The endophytic bacterium Lysobacter firmicutimachus strain 5-7 is a promising biocontrol agent against rice seedling disease caused by Pythium arrhenomanes in nursery trays. Plant Dis. doi: 10.1094/PDIS-05-22-1195-RE
van der Plaats-Niterink AJ (1981) Monograph of the genus Pythium. Studies in Mycology N°21, 1–242.
van West P, Morris BM, Reid B, et al. (2002) Oomycete plant pathogens use electric fields to target roots. Molecular Plant Microbe Interactions 15(8): 790-8. doi: 10.1094/MPMI.2002.15.8.790
van West P, Appiah AA, Gow NA (2003) Advances in research on oomycete root pathogens. Physiological and Molecular Plant Pathology 62: 99–113. doi: 10.1016/S0885-5765(03)00044-4
Veterano ST, Coffua LS, Mena-Ali JI, Blair JE (2017) Pythium yorkensis sp. nov., a potential soybean pathogen from southeastern Pennsylvania, USA. Plant Pathology (accepted). doi: 10.1111/ppa.12779
Wang M, Du Y, Ling C, et al. (2021) Design, Synthesis and Antifungal/Anti‐Oomycete Activity of Pyrazolyl Oxime Ethers as Novel Potential Succinate Dehydrogenase Inhibitors. Pest Manag Sci. Accepted Author Manuscript. doi: 10.1002/ps.6418
Wang N, Yin Z, Wu Y, et al. (2023) A Pythium myriotylum Small Cysteine-Rich Protein Triggers Immune Responses in Diverse Plant Hosts. Mol Plant Microbe Interact.: MPMI09220187R. doi: 10.1094/MPMI-09-22-0187-R
Zitnick-Anderson KK, Nelson BDJr (2015) Identification and pathogenicity of Pythium on soybean in North Dakota. Plant Disease 99(1): 31-38. doi: 10.1094/PDIS-02-14-0161-RE