.
Condición fitosanitaria: Presente
Grupo de cultivos: Cereales
Rango de hospedantes: muy específico / estrecho
Hospedante primario o agronómico (estado uredinial/telial): trigo común (Triticum aestivum L.), trigo duro (T. turgidum L. var. durum), trigo emmer cultivado (T. dicoccon) y trigo emmer silvestre (T. dicoccoides), Aegilops speltoides, pasto de cabra (Ae. cylindrica), y triticale (X Triticosecale) (estadios telial/uredinial) (Roelfs et al., 1992; Bolton et al., 2008)
Hospedante intermediario (estado pycnial/aecial): Thalictrum speciosissimum (= T. flavum glaucum) y Isopyrum fumaroides (estadios pycnial / aecial) (Roelfs et al., 1992; Bolton et al., 2008)
Epidemiología: policíclica, subaguda.
Ciclo: macrocíclica, heteroica
Etiología: Hongo. Biotrófico
Agente causal: Puccinia triticina Erikss. 1899 (Pt) (ex Puccinia recondita f. sp. tritici)
Taxonomía: Fungi > Basidiomycota > Pucciniomycotina > Pucciniomycetes > Pucciniales > Pucciniaceae > Puccinia
.
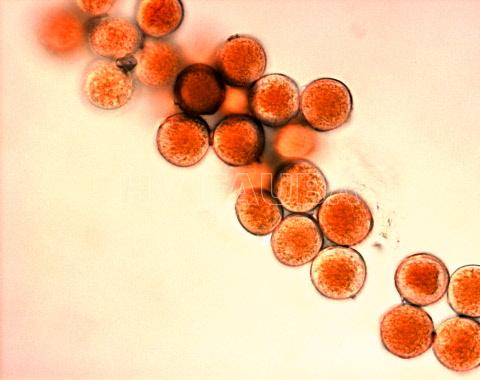
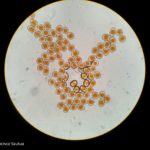
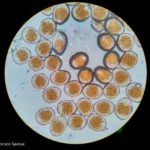
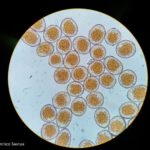
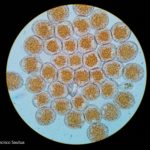
- Uredosporas de Puccinia triticina. Autor: Geagea et al., 1999
.
.
Antecedentes e importancia económica
La roya anaranjada o de la hoja del tirgo causada por Puccinia triticina Erikss. se encuentra en casi todos los lugares donde se cultiva trigo en condiciones no áridas (Saari y Prescott, 1985; Samborski, 1985), y es la principal causa de pérdida de rendimiento en el trigo debido a enfermedades a nivel mundial (Savary et al., 2019).
.
Síntomas y signos
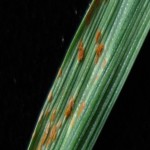
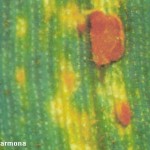
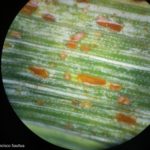
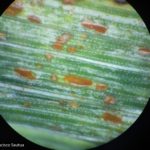
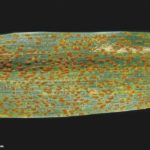
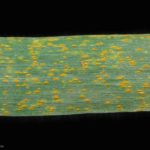
Los síntomas pueden manifestarse en todos los órganos verdes de la planta. Surgen pequeñas pústulas (uredias) redondeadas de color anaranjado (vivaces) dispuestas sin orden sobre el haz superior de las hojas y se extienden hacia las vainas. En el interior de las uredias se producen las esporas denominadas urediniosporas de alrededor de 1.5 mm de diámetro. A estas pústulas le siguen los teleutosoros y sus correspondientes teleutosporas de color negro. Estas fructificaciones quedan cubiertas por la epidermis hasta el final del ciclo de la planta.
.
Ciclo de la enfermedad, Epidemiología
El patógeno sobrevive parasitando plantas de trigo voluntarias (guachas). El viento es el principal mecanismo de diseminación de las urediniosporas. La roya anaranjada es la más común de todas y puede comenzar su infección desde la aparición de las primeras hojas. La temperatura óptima es de 16 a 18ºC, con 10 horas de mojado. Es la roya que virtualmente se presenta en todas las regiones trigueras. La diseminación por el viento es muy eficiente. El patrón de distribución en el lote es generalizada y uniforme.
.
Condiciones predisponentes:
* Para el establecimiento de la enfermedad (infecciones primarias): Temperaturas del alrededor de 20 ° C y formación de rocío durante varias horas.
* Para la dispersión de la enfermedad (infecciones secundarias): Días soleados y ventosos, temperaturas de alrededor de 20 ° C y noches con formación de rocío durante varias horas.
.
Factores de Riesgo:
* Uso de cultivares susceptibles,
* Tiempo relativamente templado y húmedo
* Temperaturas por encima de la normal hacia fines de invierno (15 de agosto al 15 de setiembre)
* Excesiva fertilización nitrogenada
* Alta densidad de plantas.
.
Daños
La roya anaranjada es la roya más común que afecta el cultivo del trigo y la más plástica en requerimientos de temperaturas. Por esto, ocurre en todas las regiones trigueras.Su importancia relativa es de moderada a alta. Las plantas afectadas producen granos chuzos. En ataques tempranos e intensos producen menor número de granos/espiga.
.
La etapa sexual de P. triticina rara vez se encuentra en Thalictrum spp. en América del Norte (Levine y Hildreth, 1957). Se ha encontrado en T. speciosissimum en el sur de Europa (Casulli y Siniscalco, 1987; D’Oliveira, 1940; D’Oliveira y Samborski, 1966; Young y D’Oliveira, 1982). Las infecciones del hospedante alternativo I. fumaroides parecen estar restringidas a una región de Siberia (Chester, 1946). En Argentina, no hay estudios actualizados sobre este aspecto de la enfermedad.
.
Manejo de la enfermedad
Debido a sus características epidemiológicas, las rotaciones o el manejo de los rastrojos no son medidas adecuadas.
.
Medidas preferenciales de manejo
* Uso de cultivares resistentes: es la medida más efectiva, aunque la resistencia de ciertos cultivares puede “quebrarse”.
* Eliminación de plantas guachas: es importante porque reduce el inóculo en un lote, pero por sí sola no es suficiente.
* Aplicación de fungicidas cuando se alcance el UDE (umbral de daño económico).
.
.
- 1 Síntomas de Roya anaranjada o de la hoja (Puccinia triticina Eriks)
- 2 Síntomas de Roya anaranjada o de la hoja (Puccinia triticina Eriks)
- 3 Uredinosoros de Roya anaranjada o de la hoja (Puccinia triticina Eriks)
- 4 Uredinosoros de Roya anaranjada o de la hoja (Puccinia triticina Eriks)
- 5 Uredinosoros de Roya anaranjada o de la hoja (Puccinia triticina Eriks)
- 6 Uredinosporas de Roya anaranjada o de la hoja (Puccinia triticina Eriks)
- 7 Uredinosporas de Roya anaranjada o de la hoja (Puccinia triticina Eriks)
- 8 Uredinosporas de Roya anaranjada o de la hoja (Puccinia triticina Eriks)
- 9 Uredinosporas de Roya anaranjada o de la hoja (Puccinia triticina Eriks)
- Uredinosoros de Puccinia triticina. Autor: Dirceu Gassen
- Uredinosoros de Puccinia triticina. Autor: Dirceu Gassen
- Uredinosoros de Puccinia triticina. Autor: Dirceu Gassen
- Autor: Dirceu Gassen
- Teliosoros de P. triticina en hoja de trigo. Autor: Dirceu Gassen
.
Video
Rust: the fungi that attacks plants. Created by Chris Hammang, Producer Sean O’Donoghue, Scientific Consultant Peter Dodds. C SIRO (Video)
.
.
Bibliografía
Aktar-Uz-Zaman Md, Tuhina-Khatun Mst, Hanafi MM, Sahebi M (2017) Genetic analysis of rust resistance genes in global wheat cultivars: an overview. Biotechnology & Biotechnological Equipment 31:3: 431-445. doi: 10.1080/13102818.2017.1304180
Aoun M, Kolmer JA, Rouse MN, et al. (2017) Inheritance and Bulked Segregant Analysis of Leaf Rust and Stem Rust Resistance in Durum Wheat Genotypes. Phytopathology 107(12): 1496-1506. doi: 10.1094/PHYTO-12-16-0444-R
Aoun M, Kolmer JA, Breiland M (2020) Genotyping-by-Sequencing for the Study of Genetic Diversity in Puccinia triticina. Plant Disease 104: 752-760. doi: 10.1094/PDIS-09-19-1890-RE
Arora S, Kaur S, Dhillon GS, et al. (2021) Introgression and genetic mapping of leaf rust and stripe rust resistance in Aegilops triuncialis. J Genet 100: 6. doi: 10.1007/s12041-020-01253-3
, , (2022) Contribution of the life-history traits of a plant pathogen to the development of field epidemics. Plant Pathology 71: 1344– 1354. doi: 10.1111/ppa.13567
Babayants O, Babayants L, Gorash A, et al. (2015) Physiologic specialization of Puccinia triticina Erikss. and effectiveness of Lr-genes in the south of Ukraine during 2013-2014. Chilean journal of agricultural research 75(4): 443-450. doi: 10.4067/S0718-58392015000500009
Babu P, Baranwal DK, Harikrishna, et al. (2020) Application of Genomics Tools in Wheat Breeding to Attain Durable Rust Resistance. Frontiers in Plant Science 11: 567147. doi: 10.3389/fpls.2020.567147
Badar R, Ahmed A, Batool S, et al. (2023) Puccinia triticina: wheat stripe (yellow) rust pathogen: a review. Pakistan Journal of Phytopathology 35(2): 473-480. doi: 10.33866/phytopathol.035.02.0889
Balint-Kurti P (2019) The plant hypersensitive response: concepts, control and consequences. Molecular Plant Pathology 20: 1163-1178. doi: 10.1111/mpp.12821
Baranwal D (2022) Genetic and genomic approaches for breeding rust resistance in wheat. Euphytica 218: 159. doi: 10.1007/s10681-022-03111-y
Bhardwaj SC, Singh GP, Gangwar OP, et al. (2019) Status of Wheat Rust Research and Progress in Rust Management-Indian Context. Agronomy 9 (12): 1–14. doi: 10.3390/agronomy9120892
Bockus WW, Bowden RL, Hunger RM, Murray TD, Smiley RW, Morrill W (eds.) (2010) Compendium of Wheat Diseases and Insects, Third Edition. APS Press, Minneapolis
Bolton MD, Kolmer JA, Garvin DF (2008) Wheat leaf rust caused by Puccinia triticina. Molescular Plant Pathology 9(5): 563-75. doi: 10.1111/j.1364-3703.2008.00487.x
Bolton MD, Kolmer JA, Xu WW, Garvin DF (2008) Lr34-mediated leaf rust resistance in wheat: transcript profiling reveals a high energetic demand supported by transient recruitment of multiple metabolic pathways. Molecular Plant Microbe Interactions 21(12): 1515-1527. doi: 10.1094/MPMI-21-12-1515
Bruce M, Neugebauer KA, Joly DL, et al (2014) Using transcription of six Puccinia triticina races to identify the effective secretome during infection of wheat. Front. Plant Sci. 4: 520. doi: 10.3389/fpls.2013.00520
Campos PE, Cardarelli FO (2023) Situación actual de las royas de trigo en Argentina. Cambios en las poblaciones de los patógenos y comportamiento sanitario de los cultivares de trigo. INTA. Link
Carmona M, Sugía V, Jaeggi E, Reis EM (2004) Roya de la hoja de trigo (Puccinia triticina): estimación de daños y pérdidas, y su relación con el control químico como estrategia racional y económica. Fitopatología Brasilera 29: 90.
Casassola A, Ereful NC, Zanella CM, et al. (2024) Transcriptional profiling identifies the early responses to Puccinia triticina infection in the adult plant leaf rust resistant wheat variety Toropi. Plant Pathology 00: 1–14. doi: 10.1111/ppa.13865
(1987) Thalictrum flavum L. as an alternate host of Puccinia recondita f. sp. tritici in southern Italy. In: 7th Congress of the Mediterranean Phytopathology Union, Granada, Spain.
(1946) The Nature and Prevention of the Cereal Rusts as Exemplified in the Leaf Rust of Wheat. Waltham, MA: Chronica Botanica.
Chhetri M, Bariana H, Wong D, et al. (2017) Development of robust molecular markers for marker-assisted selection of leaf rust resistance gene Lr23 in common and durum wheat breeding programs. Molecular Breeding 37: 21. doi: 10.1007/s11032-017-0628-6
Dean R, Van Kan JAL, Pretorius ZA, et al. (2012) The Top 10 fungal pathogens in molecular plant pathology. Molecular Plant Pathology 13: 414–430. doi: 10.1111/j.1364-3703.2011.00783.x
Dehkordi HR, El Jarroudi M, Kouadio L, et al. (2020) Monitoring Wheat Leaf Rust and Stripe Rust in Winter Wheat Using High-Resolution UAV-Based Red-Green-Blue Imagery. Remote Sensing 12: 3696. doi: 10.3390/rs12223696
Dmochowska-Boguta M, Alaba S, Yanushevska Y, et al. (2015) Pathogen-regulated genes in wheat isogenic lines differing in resistance to brown rust Puccinia triticina. BMC Genomics 16: 742. doi: 10.1186/s12864-015-1932-3
(1940) Notas sobre a produçao da fase aecidica de algumas ferrugens dos cereais em Portugal. Revista Agronomica 28: 201–208.
(1966) Aecial stage of Puccinia recondita on ranunculaceae and boraginaceae in portugal. In: Proceedings of the first European Brown Rust Conference (Macer, R.C. and Wolfe, M.S., eds), pp. 133–150. Cambridge, UK.
Eriksson J, Henning E (1896) The cereal rusts. Nortstedt & Söner, Stockholm.
Gao P, Zhou Y, Gebrewahid TW, et al. (2019) Identification of known leaf rust resistance genes in common wheat cultivars from Sichuan province in China. Crop Protection 115: 122-129. doi: 10.1016/j.cropro.2018.09.012
Gaurav K, Arora S, Silva P, et al. (2021) Population genomic analysis of Aegilops tauschii identifies targets for bread wheat improvement. Nat Biotechnol. doi: 10.1038/s41587-021-01058-4
Geagea L, Huber L, Sache I (1999) Dry-dispersal and rain-splash of brown (Puccinia recondita f. sp. tritici) and yellow (P. striiformis) rust spores from infected wheat leaves exposed to simulated raindrops. Plant Pathology 48: 472-482. Link
Gebrewahid TW, Yao Z-J, Yan X-C, Gao P, Li Z-F (2017) Identification of Leaf Rust Resistance Genes in Chinese Common Wheat Cultivars. Plant Disease 101(10): 1729-1737. doi: 10.1094/PDIS-02-17-0247-RE
Gu J, Sun J, Liu N, et al. (2020) A novel cysteine-rich receptor-like kinase gene, TaCRK2, contributes to leaf rust resistance in wheat. Mol Plant Pathol. 21(5): 732-746. doi: 10.1111/mpp.12929
Gultyaeva EI, Aristova MK, Shaidayuk EL, et al. (2017) Genetic differentiation of Puccinia triticina Erikss. in Russia. Russian Journal of Genetics 53(9): 998–1005. doi: 10.1134/S1022795417070031
Gupta N, Bhardwaj SC, Sharma TR, et al. (2018) Population behaviour of predominant and virulent pathotypes of Puccinia triticina causing wheat brown rust in India. Indian Phytopathology 1–6. doi: 10.1007/s42360-018-0009-z
Hanzalová A, Bartoš P, Sumíková T (2016) Virulence of wheat leaf rust (Puccinia triticina Eriks.) in the years 2013–2015 and resistance of wheat cultivars in Slovakia. Cereal Research Communications 44(4): 585-593. doi: 10.1556/0806.44.2016.030
Hanzalová A, Bartoš P, Sumíková T (2017) Pathotypes of Wheat Leaf Rust (Puccinia triticina Eriks.) and Resistance of Registered Cultivars in the Czech Republic in 2012–2015. Czech Journal of Genetics and Plant Breeding 53(3): 122–126. doi: 10.17221/121/2016-CJGPB
Hewitt T, Zhang J, Huang L, et al. (2021) Wheat leaf rust resistance gene Lr13 is a specific Ne2 allele for hybrid necrosis. Mol Plant. 14(7): 1025-1028. doi: 10.1016/j.molp.2021.05.010
Hovmøller MS, Thach T, Justesen AF (2023) Global dispersal and diversity of rust fungi in the context of plant health. Current Opinion in Microbiology 71: 102243. doi: 10.1016/j.mib.2022.102243
Hu G, Linning R, McCallum B, et al. (2007) Generation of a wheat leaf rust, Puccinia triticina, EST database from stage-specific cDNA libraries. Molecular Plant Pathology 8(4): 451-67. doi: 10.1111/j.1364-3703.2007.00406.x
Iqbal M, Semagn K, Jarquin D, et al. (2022) Identification of Disease Resistance Parents and Genome-Wide Association Mapping of Resistance in Spring Wheat. Plants 11(21): 2905. doi: 10.3390/plants11212905
Imbaby IA, Mahmoud MA, Hassan MEM, et al. (2014) Identification of Leaf Rust Resistance Genes in Selected Egyptian Wheat Cultivars by Molecular Markers. The Scientific World Journal. 2014: 574285. doi: 10.1155/2014/574285
Kassa MT, You FM, Hiebert CW, et al. (2017) Highly predictive SNP markers for efficient selection of the wheat leaf rust resistance gene Lr16. BMC Plant Biology 17(1): 45. doi: 10.1186/s12870-017-0993-7
Kiran K, Rawal HC, Dubey H, et al. (2016) Draft Genome of the Wheat Rust Pathogen (Puccinia triticina) Unravels Genome-Wide Structural Variations during Evolution. Genome Biology and Evolution 8(9): 2702-271. doi: 10.1093/gbe/evw197
Kolmer J (2013) Leaf Rust of Wheat: Pathogen Biology, Variation and Host Resistance. Forests 4(1): 70-84. doi: 10.3390/f4010070
Kolmer JA, Hanzalova A, Goyeau H, Bayles R, Morgounov A (2013) Genetic differentiation of the wheat leaf rust fungus Puccinia triticina in Europe. Plant Pathology 62(1): 21–31. doi: 10.1111/j.1365-3059.2012.02626.x
Kolmer JA, Mirza JI, Imtiaz M, Shah SJA (2017) Genetic Differentiation of the Wheat Leaf Rust Fungus Puccinia triticina in Pakistan and Genetic Relationship to Other Worldwide Populations. Phytopathology 107(6): 786-790. doi: 10.1094/PHYTO-10-16-0388-R
Kolmer JA, Herman A, Ordoñez ME, et al. (2020) Endemic and panglobal genetic groups, and divergence of host-associated forms in worldwide collections of the wheat leaf rust fungus Puccinia triticina as determined by genotyping by sequencing. Heredity 124, 397–409. doi: 10.1038/s41437-019-0288-x
, , , et al (2024) Influence of biotic and abiotic factors on the virulence of Puccinia triticina population in southern Russia. Plant Pathology 73: 404–418. doi: 10.1111/ppa.13816
Kuzdraliński A, Kot A, Szczerba H, et al. (2017) Novel PCR Assays for the Detection of Biological Agents Responsible for Wheat Rust Diseases: Puccinia triticina and Puccinia striiformis f. sp. tritici. Journal of Molecular Microbiology and Biotechnology 27:299-305. doi: 10.1159/000481799
Levine M, Hildreth RC (1957) A natural occurrence of the aecial stage of Puccinia rubigo-vera var. tritici in the United States. Phytopathology 47: 110–111.
Li F, Li Y, Cao L, et al. (2018) Simultaneous Transfer of Leaf Rust and Powdery Mildew Resistance Genes from Hexaploid Triticale Cultivar Sorento into Bread Wheat. Frontiers in Plant Science 9: 85. doi: 10.3389/fpls.2018.00085
Lintz J, Dubrulle G, Cawston E, et al. (2022) A Short Review of Anti-Rust Fungi Peptides: Diversity and Bioassays. Front. Agron. 4: 966211. doi: 10.3389/fagro.2022.966211
Lu Y, Bowden RL, Zhang G, et al. (2017) Quantitative Trait Loci for Slow-Rusting Resistance to Leaf Rust in Doubled-Haploid Wheat Population CI13227 × Lakin. Phytopathology 107(11): 1372-1380. doi: 10.1094/PHYTO-09-16-0347-R
Manjunatha C, Sharma S, Kulshreshtha D, et al. (2018) Rapid detection of Puccinia triticina causing leaf rust of wheat by PCR and loop mediated isothermal amplification. PLoS ONE 13(4): e0196409. doi: 10.1371/journal.pone.0196409
Matthew J. Moscou, H. Peter Van Esse (2017) The quest for durable resistance. Science 358(6370): 1541-1542. doi: 10.1126/science.aar4797
McCallum BD, Hiebert CW, Cloutier S, et al. (2016) A review of wheat leaf rust research and the development of resistant cultivars in Canada. Canadian Journal of Plant Pathology 38(1): 1-18. doi: 10.1080/07060661.2016.1145598
MCcallum BD, Seto-Goh P, Xue A (2016) Physiologic specialization of Puccinia triticina, the causal agent of wheat leaf rust, in Canada in 2010. Canadian Journal of Plant Pathology 38(4): 440-447. doi: 10.1080/07060661.2016.1261047
McCallum BD, Seto-Goh P, Foster A, Xue A (2018) Physiological specialization of Puccinia triticina, the causal agent of wheat leaf rust, in Canada in 2012. Canadian Journal of Plant Pathology. doi: 10.1080/07060661.2018.1495267
Mojerlou S, Moeller M, Schwessinger B, Rodriguez-Algaba J (2024) Beyond Asexual: Genomics-Driven Progress in Unveiling Sexual Reproduction in Cereal Rust Fungi. Mol Plant Microbe Interact. doi: 10.1094/MPMI-10-24-0122-FI
Norman M, Bariana H, Bansal U, Periyannan S (2023) The Keys to Controlling Wheat Rusts: Identification and Deployment of Genetic Resistance. Phytopathology 113(4): 667-677. doi: 10.1094/PHYTO-02-23-0041-IA
Nyamesorto B, Zhang H, Rouse M, et al. (2022) A transcriptomic-guided strategy used in identification of a wheat rust pathogen target and modification of the target enhanced host resistance to rust pathogens. Front. Plant Sci. 13: 962973. doi: 10.3389/fpls.2022.962973
Omara RI, Abdelaalb KAA (2018) Biochemical, histopathological and genetic analysis associated with leaf rust infection in wheat plants (Triticum aestivum L.). Physiological and Molecular Plant Pathology 104: 48-57. doi: 10.1016/j.pmpp.2018.09.004
Ordoñez ME, Germán SE, Kolmer JA (2010) Genetic differentiation within the Puccinia triticina population in South America and comparison with the North American population suggests common ancestry and intercontinental migration. Phytopathology 100: 376-383. doi: 10.1094/PHYTO-100-4-0376
Panwar V, Jordan M, McCallum B, Bakkeren G (2017) Host-induced silencing of essential genes in Puccinia triticina through transgenic expression of RNAi sequences reduces severity of leaf rust infection in wheat. Plant Biotechnology Journal (in press). doi: 10.1111/pbi.12845
Peng FY, Yang RC (2017) Prediction and analysis of three gene families related to leaf rust (Puccinia triticina) resistance in wheat (Triticum aestivum L.). BMC Plant Biology 17: 108. doi: 10.1186/s12870-017-1056-9
Pinto da Silva GB, Zanella CM, Martinelli JA, et al. (2018) Quantitative Trait Loci Conferring Leaf Rust Resistance in Hexaploid Wheat. Phytopathology (accepted). doi: 10.1094/PHYTO-06-18-0208-RVW
Prahl KC, Klink H, Hasler M, et al. (2022) Can Decision Support Systems Help Improve the Sustainable Use of Fungicides in Wheat? Sustainability 14(23): 15599. doi: 10.3390/su142315599
Prasad PB, Bhardwaj SC, Gangwar OP, et al. (2017) Population differentiation of wheat leaf rust fungus Puccinia triticina in South Asia. Current Science 112(10): 2073-2084. doi: 10.18520/cs/v112/i10/2073-2084
Qureshi N, Bariana H, Kolmer JA, et al. (2017) Genetic and Molecular Characterization of Leaf Rust Resistance in Two Durum Wheat Landraces. Phytopathology 107(11): 1381-1387. doi: 10.1094/PHYTO-01-17-0005-R
Rahmatov M, Otambekova M, Muminjanov H, et al. (2019) Characterization of stem, stripe and leaf rust resistance in Tajik bread wheat accessions. Euphytica 215: 55. doi: 10.1007/s10681-019-2377-6
Rampitsch C, Huang M, Djuric-Cignaovic S, Wang X and Fernando U (2019) Temporal Quantitative Changes in the Resistant and Susceptible Wheat Leaf Apoplastic Proteome During Infection by Wheat Leaf Rust (Puccinia triticina). Front. Plant Sci. 10:1291. doi: 10.3389/fpls.2019.01291
Roelfs AP (1985) Wheat and rye stem rust. pp. 3–37. In: Roelfs, A. P., Bushnell, W. R. (eds.), The Cereal Rusts Vol. II: Diseases, Distribution, Epidemiology, and Control. Orlando, FL: Academic.
Roelfs AP, Singh RP, Saari EE (1992) Rust Diseases of Wheat: Concepts and methods of disease management. Mexico, D.F.: CIMMYT. 81 pages.
Różewicz M, Wyzińska M, Grabiński J (2021) The Most Important Fungal Diseases of Cereals—Problems and Possible Solutions. Agronomy 11(4): 714. doi: 10.3390/agronomy11040714
Saari EE, Young HC, Kernkamp MF (1968) Infection of North American Thalictrum spp. with Puccinia recondita f. sp. tritici. Phytopathology 58: 939–943.
Saari EE, Prescott JM (1985) World distribution in relation to economic losses. In: Roelfs AP, Bushnell WR (eds) The Cereal Rusts. Academic Press, Orlando, p 259–298.
Samborski DJ (1985) Wheat leaf rust. In: Roelfs AP, Bushnell WR (eds) The Cereal Rusts. Academic Press, Orlando, Fla., p 39–59. vol 2.
Savadi S, Prasad P, Kashyap PL, Bhardwaj SC (2017) Molecular breeding technologies and strategies for rust resistance in wheat (Triticum aestivum) for sustained food security. Plant Pathology 67: 771-791. doi: 10.1111/ppa.12802
Savadi S, Prasad P, Sharma K, et al. (2002) Development of novel transcriptome-based SSR markers in Puccinia triticina and their potential application in genetic diversity studies. Tropical Plant Pathology 45: 499–510. doi: 10.1007/s40858-020-00347-8
Savary S, Willocquet L, Pethybridge SJ, et al. (2019) The global burden of pathogens and pests on major food crops. Nat Ecol Evol 3: 430–439. doi: 10.1038/s41559-018-0793-y
Semagn K, Iqbal M, Jarquin D, et al. (2022) Genomic Predictions for Common Bunt, FHB, Stripe Rust, Leaf Rust, and Leaf Spotting Resistance in Spring Wheat. Genes 13(4): 565. doi: 10.3390/genes13040565
Serfling A, Templer SE, Winter P, Ordon F (2016) Microscopic and Molecular Characterization of the Prehaustorial Resistance against Wheat Leaf Rust (Puccinia triticina) in Einkorn (Triticum monococcum). Frontiers in Plant Science 7: 1668. doi: 10.3389/fpls.2016.01668
Singh RP, Huerta-Espino J, Roelfs AP (2002) The wheat rusts. In: Curtis BC, Rajaram S, Gómez Macpherson H (Eds.) Bread wheat. FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS, Rome. FAO Plant Production and Protection Series 30
Sisson AJ, Allen T, Andersen K, et al. (2025) Estimated yield reductions and economic losses on wheat caused by disease from 2018 through 2021. Plant Health Progress. doi: 10.1094/PHP-09-24-0087-RS
Skolotneva ES, Leonova IN, Bukatich EY, et al. (2018) Effectiveness of leaf rust resistance genes against Puccinia triticina populations in Western Siberia during 2008–2017. Journal of Plant Diseases and Protection (online): 1–7. doi: 10.1007/s41348-018-0191-3
Sperschneider J, Hewitt T, Lewis DC, et al. (2023) Nuclear exchange generates population diversity in the wheat leaf rust pathogen Puccinia triticina. Nat Microbiol. doi: 10.1038/s41564-023-01494-9
Sucher J, Menardo F, Praz CR, et al. (2017) Transcriptional profiling reveals no response of fungal pathogens to the durable, quantitative Lr34 disease resistance gene of wheat. Plant Pathology (in press). doi: 10.1111/ppa.12797
Suffert F (2024) Disruptive effect of rainfalls on the diurnal periodicity of airborne wheat rust spore under field conditions. bioRxiv 2024.10.03.616546; doi: 10.1101/2024.10.03.616546
Talebi R, Mahboubi M, Naji AM, et al. (2023) Physiological specialization of Puccinia triticina and genome-wide association mapping provide insights into the genetics of wheat leaf rust resistance in Iran. Sci Rep 13: 4398. doi: 10.1038/s41598-023-31559-y
Talajoor M, Wang X, Zhang H, et al. (2016) Wheat mutant MNR220 delays haustoria formation during leaf rust pathogenesis at the seedling stage. Canadian Journal of Plant Pathology 38(3): 338-347. doi: 10.1080/07060661.2016.1205663
Wang MN, Wan AM, Chen XM (2015) Barberry as alternate host is important for Puccinia graminis f. sp. tritici but not for Puccinia striiformis f. sp. tritici in the U.S. Pacific Northwest. Plant Disease 99: 1507-1516. doi: 10.1094/PDIS-12-14-1279-RE
, , , et al (2023) The analysis of Puccinia triticina field populations in Canada between 2018 and 2020 using restriction site-associated DNA genotyping-by-sequencing. Plant Pathology 00: 1–13. doi: 10.1111/ppa.13805
Wu JQ, Sakthikumar S, Dong C, et al. (2017) Comparative Genomics Integrated with Association Analysis Identifies Candidate Effector Genes Corresponding to Lr20 in Phenotype-Paired Puccinia triticina Isolates from Australia. Frontiers in Plant Science 8: 148. doi: 10.3389/fpls.2017.00148
Xia C, Qiu A, Wang M, et al. (2022) Current Status and Future Perspectives of Genomics Research in the Rust Fungi. International Journal of Molecular Sciences 23(17): 9629. doi: 10.3390/ijms23179629
(1982) A further study of race populations of Puccinia recondita f. sp. tritici, Garcia de Orta, Sér. Est. Agron. Lisboa, 9, 37–52.
Zhang P, Lan C, Singh RP, et al. (2022) Identification and Characterization of Resistance Loci to Wheat Leaf Rust and Stripe Rust in Afghan Landrace “KU3067”. Front. Plant Sci. 13: 894528. doi: 10.3389/fpls.2022.894528
Zhao J, Wang M, Chen X, Kang Z (2016) Role of Alternate Hosts in Epidemiology and Pathogen Variation of Cereal Rusts. Annual Review of Phytopathology 54: 207-228. doi: 10.1146/annurev-phyto-080615-095851